Adhesive Restorations On Primary Teeth Introduction
Restorative materials can either be rigid materials or plastic materials. Plastic materials include conventional glass ionomer cement (GIC), conventional composites, and modified forms of the same.
Table of Contents
Rigid materials like amalgam are being replaced by plastic materials in current practice. Many reasons are attributed to the preference for plastic materials over rigid materials.
1. Conservative cavity preparation: Extension for prevention rule is not adopted for plastic restoration as only the carious structure is excavated. The rule of thumb, in this case, is ‘constriction with conviction’. It implies constriction of the cavity prepared with a conviction to preserve the tooth structure.
Read And Learn More: Paediatric Dentistry Notes
Direct (chemical) bonding plastic or adhesive materials do not require the removal of the intact tooth structure. While materials that bond micromechanically or composite resins require minor bevelling of enamel at the exterior cavo surface line angle.
2. Shorter treatment time: Relatively less time is required for conservative cavity preparation. Plastic materials have a shorter setting time or set on command (light cure), which complies with the short attention span of children.
3. Aesthetics: Plastic materials are tooth-colored as compared to rigid materials like amalgam. They are also supplied with shade specifications. Composite resins are more aesthetic than GICs as they simulate the color of enamel. Glass ionomers simulate the color of dentin.
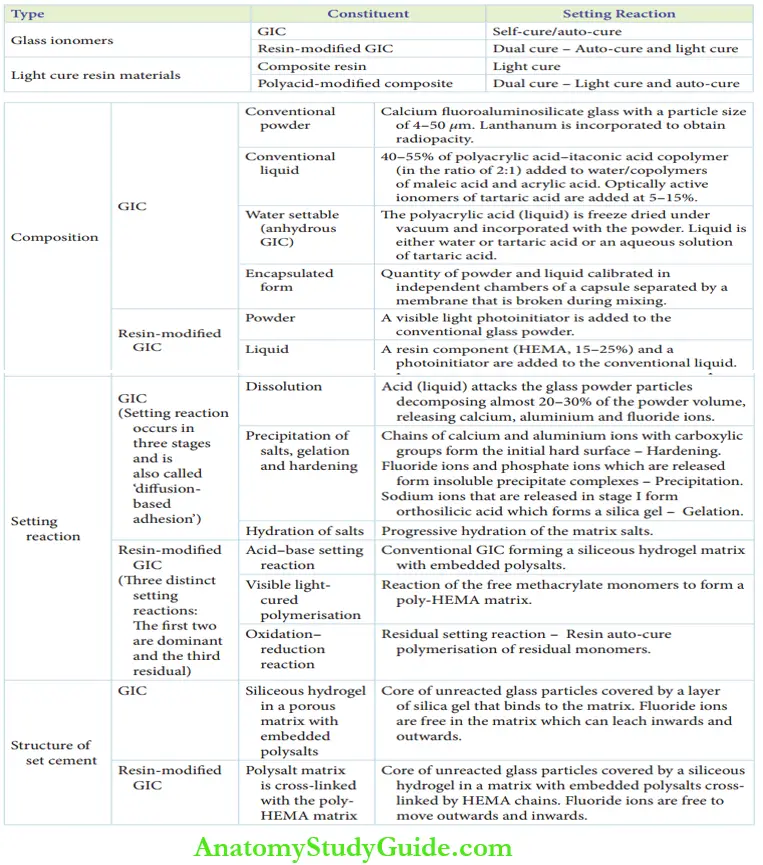
Glass Ionomer Cement
GICs were formulated by Wilson and Kent in 1973. GJ Mount describes glass ionomer as a material in which ion-leachable fluoride-containing calcium aluminum fluorosilicate glass powder reacts with polyalkenes acids in an irreversible acid–base reaction to complete the setting process.
They have been referred to as polyalkenoates. GIC can be of two types:
- Conventional GIC (self-cure/auto-cure GIC)
- Resin-modified GIC
- Light cure composite resin
- Polyacid-modified composite resin (dual cure –light cure and auto-cure)
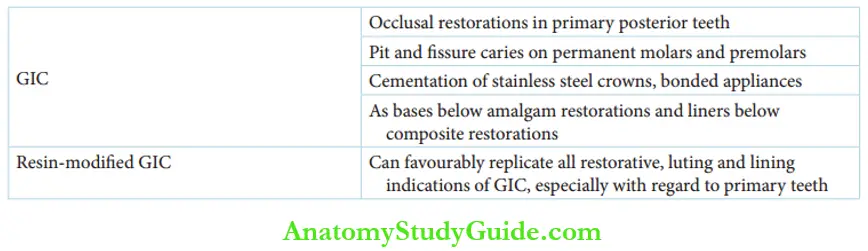
Glass Ionomer Cement Indications
GICs are indicated in the following cases:
- Occlusal restorations on primary posterior teeth
- Pit and fisure caries on permanent molars and premolars
- Cementation of stainless steel crowns and bonded appliances
- The base below amalgam restorations and liners below composite restorations (sandwich technique)
Resin-modified GIC can be favorably utilized for all restorative indications of GIC, especially with regard to primary teeth.
1. Conventional Gic (Self-Cure Gic)
GICs are manufactured and marketed in three forms, the more common form of dispensing being the first one.
- Conventional GIC – powder/liquid form
- Anhydrous GIC – powder/tartaric acid or water form
- Encapsulated form
The conventional GIC powder consists of calcium for aluminosilicate glass with a particle size of 4–50 µm. Smaller particle sizes are used for lining and cementation purposes. Coarser particles have increased translucency and are suitable for restorations.
Lanthanum or strontium is incorporated instead of calcium in the powder to obtain the radiopacity of the set cement. The ratio of minimum oxide to silicon oxide is crucial and should be >1:2 for cement formation to occur.
The major and minor constituents of the conventional GIC powder and their functions are depicted.
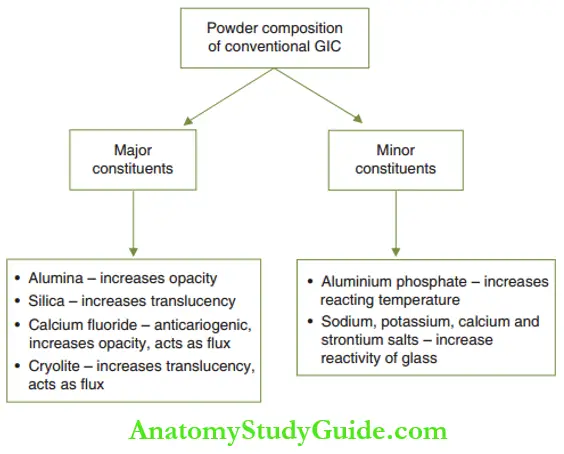
The composition of conventional GIC powder can be modified with the addition of ceramic and metal particles.
1. Ceramic metal reinforced – cermet powder:
- The composition of conventional GIC powder is modified with the addition of ceramic and metal reinforcer particles by sintering.
- The metal reinforcer can be silver, silver amalgam alloy, and silver palladium alloy.
- Titanium oxide (up to 5% by weight) is also added to impart clinically acceptable color to the material.
2. Miracle mix:
- An alternative method is to mix silver amalgam alloy flakes or spheres with conventional powder.
- The reinforcement with gold/silver metals, ceramic particles, or amalgam alloy flukes/spheres may improve the abrasion resistance of conventional GICs.
- The liquid composition of conventional GIC includes 40–55% of polyacrylic acid–itaconic acid copolymer added to water in a ratio of 2:1.
- Copolymers of malic acid and acrylic acid can be alternated with water to improve storage properties as aqueous solutions tend to become viscous with time.
Optically active ionomers of tartaric acid are added at 5–15% to the liquid to achieve the following advantages:
- Extract ions from the glass powder
- Enhance the working time
- Sharpen the setting time
- Improve the aesthetic characteristics of the set cement
Anhydrous GIC
The term is anhydrous indicates ‘no water’. Anhydrous GIC is a misnomer as it is also water-based cement. The powder component is the same as conventional GIC and the liquid is either water or tartaric acid or an aqueous solution of tartaric acid.
The liquid is freeze-dried under a vacuum and incorporated with the powder. So the powder contains the liquid constituents of the anhydrous cement.
Encapsulated GIC
An encapsulated GIC is dispensed in capsules. The capsule has two compartments. The powder and freeze-dried liquid are in one compartment. The other compartment contains water.
The two compartments are divided by a membrane, which can be broken by vibration, without damage to the capsule. The vibration is incited by a vibrator that holds the capsule.
2. Resin-Modified GIC
The components of resin-modified GIC are the same as those of conventional GIC. The resin-modified GIC system contains 15–25% of an additional resin component, hydroxyethyl methacrylate (HEMA).
Traces of a few reactive chemicals and other resins are added to complete curing by an oxidation–reduction reaction. A photoinitiator (visible light) is added to the resin-modified glass ionomer system to make the powder susceptible to ambient light.
Setting Reaction Of GIC
The setting reaction of GIC is entirely an acid–base reaction. The acid particles of the liquid attack the surface of the glass powder particles, releasing calcium, aluminum, and fluoride ions.
These ions in turn cross-link the polyacid chains into a network. This mechanism can also be called ‘diffusion-based adhesion’.
1. Setting Reaction Of Self-Cure GIC
The setting reaction occurs in three stages as explained in the following text and depicted.
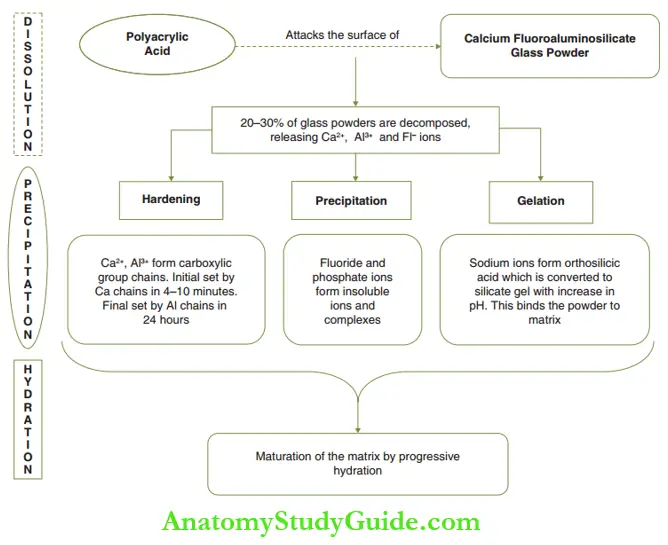
- Dissolution stage: The polyacrylic acid attacks the glass powder particles decomposing almost 20–30% of the powder volume.
- This leads to the release of calcium, aluminum, and fluoride ions, leading to the formation of a cement sol.
- The remaining 70–80% of the glass powder is called unreacted powder particles.
- Precipitation of salts: Calcium and aluminum ions bind to polyanions through carboxylic groups to form chains.
- The calcium chains form more readily creating the initial hard surface. This ‘initial set’ forms in 4–10 minutes clinically.
- The ‘final set’ is created in the next 24 hours as the less mobile aluminum ion-mediated chains are formed. This is termed hardening.
- Fluoride and phosphate ions form insoluble precipitate complexes and salts. This is referred to as precipitation.
- Sodium ions that are released initially form orthosilicic acid on the surface of the glass particles.
- When the pH rises, this acid is converted into silica gel, which binds the unreacted powder particles to the matrix. This is termed gelation.
- Hydration of salts: Maturationofthematrixsaltstakes place by progressive hydration of the matrix salts. This leads to a sharp increase in physical properties.
Setting Reaction Of GIC Structure Of The Set Cement
Cement formation will occur only when there is sufficient replacement of silicon ions by aluminum ions to render the network susceptible to acid attack. The set cement has cores of unreacted glass particles covered by a layer of silica gel binding to the matrix are depicted.
It contains cross-linked chains of carboxylic acid groups mediated by calcium and aluminum ions. Freely moving flo ride ions leach inwards and outwards in the matrix. The structure of the cement can be referred to as a ‘siliceous, hydrogel in a porous matrix with embedded polysalts’.
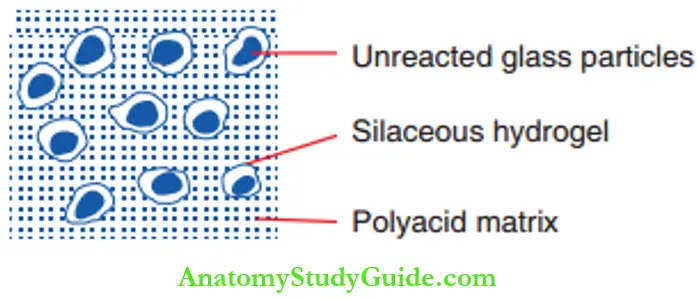
2. Setting Reaction Of Resin-Modified GIC
There are three distinct reactions in the setting process of resin-modified GIC. Of these, the first two are dominant and the third is a residual reaction. The three individual but dependent reactions are depicted.
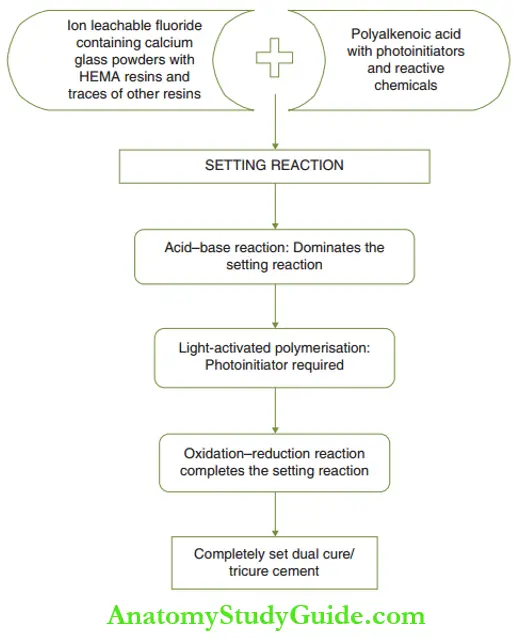
1. Acid-base setting reaction:
- The acid–base reaction has been explained under the setting reaction of self-cure GIC.
- The cement forms a siliceous hydrogel matrix with embedded polysalts.
2. Visible light cure polymerization reaction:
- A cam hydroquinone photoinitiator initiates the polymerization reaction of the free methacrylate monomers to form a poly-HEMA matrix.
- The methacrylate group replaces a small fraction of the carboxylate group in polyalkenoic acid.
- This prevents the separation of the individual matrices (poly-HEMA matrix and carboxylic acid matrix).
- Final hardening occurs by a visible light-activated cross-linkage of the polymeric chains.
3. Oxidation-reduction reaction:
- The setting is completed by auto-curing of residual monomers.
- The oxidation–reduction type of reaction mediated by reactive chemicals enhances the degree of the set.
Setting Reaction Of Resin-Modified GIC Structure Of The Set Cement
The set material contains a core of unreacted glass particles covered by a siliceous hydrogel in a matrix with embedded polysalts. This polysalt matrix is cross-linked with the poly-HEMA matrix. HEMA chains are cross-linked over the matrix are depicted.
Fluoride ions are free to move through the material as well as move outwards and inwards.
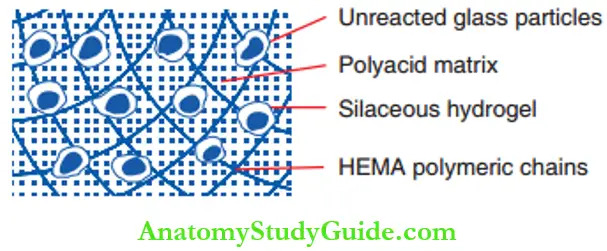
Setting Reaction Of Resin-Modified GIC Factors Influencing The Setting Reaction Of The GIC
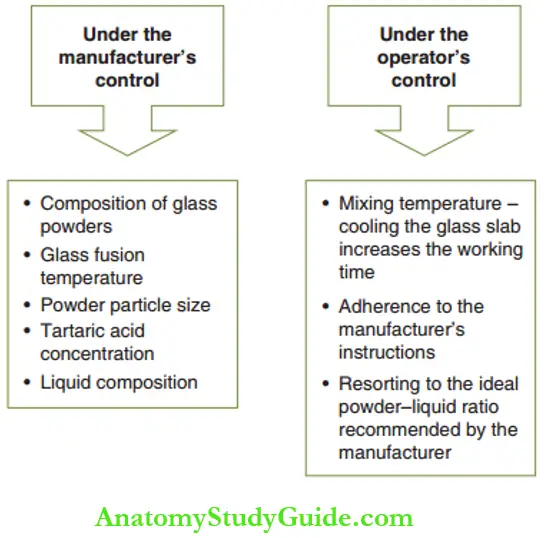
The factors influencing the setting reaction of the GIC can be controlled by the operator or the manufacturer.
1. Powder–liquid ratio (P:L):
-
- Approximately 1.8 g of powder and 1 mL of liquid is the ratio followed in most of cases.
- However, each manufacturer recommends a distinct powder: liquid (P/L) ratio and provides the corresponding dispensing spoon.
- Inappropriate P: L ratios can be very detrimental to the properties of the set glass ionomer material. Two situations can occur.
- More powder and less liquid: In this case, there is insufficient liquid to wet all the powder particles.
- This declines the translucency of the cement. Fewer ions diffuse and the volume of chains that form the matrix is poor. Silica gel is also scanty.
- There can be serious deterioration of the physical properties of the set cement.
- More liquid and less powder: In this case, the excessive liquid dissolves almost all of the powder particles leaving a negligible amount of unreacted powder particles.
- The set cement, as explained earlier, requires a core of unreacted particles at its center for it to display appreciable strength and durability.
- Hence, there is a demonstrable compromise in the physical properties of the set cement.
2. Dispensing of GICs:
-
- Glass ionomers are available in the form of capsules for mechanical mixing and in powder/liquid form for manual mixing.
- Capsule form for mechanical mixing: The capsule provides a consistent and ideal powder/liquid ratio and ensures optimal physical properties.
- The capsule also acts as a syringe for the placement of the cement into the prepared cavity.
- Different systems have varied mixing times and the operator has to adhere to the manufacturer’s recommendations.
- Powder/liquid form for manual mixing: The powder and liquid are dispensed onto a pad as per the manufacturer’s recommendations.
- Manual mixing has been explained as the next factor.
3. Steps of manipulation of cement:
The scoop of powder is divided into two equal parts. The first part of the powder is gently and rapidly rolled into the liquid within 10 seconds.
The objective of this mixing is to coat the glass particles with the ionomer liquid without dissolving it. The second part is also incorporated into the liquid in the next 15 seconds with no residue left Once mixing is completed.
The cement should have a ‘gloss and wet’ texture, which expresses the presence of unreacted carboxylic acid chains open to adhering with dentin or enamel.
The manipulation of cement onto the tooth has to be completed within a working time of 60–90 seconds. The cement can also be transferred into a disposable syringe for accurate placement.
4. Importance of isolation:
Proper isolation determines the restoration’s longevity. As explained in the setting reaction, calcium polyacrylate chains are formed initially followed by aluminium polyacrylate chains. Calcium polyacrylate chains are soluble and degrade completely in contact with water or saliva.
The dissolution of calcium polyacrylate chains leads to significant absorption of water into the cement, loss of translucency of cement, and poor physical properties of the cement.
Hence, isolation of the cavity during restoration is important to prevent deterioration of the properties of the set cement. Newer fast-set GICs have lanthanum or strontium instead of calcium.
Lanthanum or strontium polyacrylate chains are insoluble in water and hence remain unaffected by contamination with water or saliva. They are ‘water-insensitive’ and can be used in poor isolation conditions such as in an anxious pre-schooler.
5. Water content and proneness to dehydration:
- Set GIC contains 24% water.
- It is susceptible to dehydration, which can cause cracking or fissuring of the cement, softening of the surface, and loss of matrix-forming ions.
- The inherent water content has to be protected by the application of a light cure enamel bonding agent containing maleic acid.
- A generous layer has to be applied after the initial set (at 4 minutes) to ensure water stability.
- Final contouring has to be carried out after 24 hours under an air-water spray to avoid dehydration.
- The resin-modified GICs have an immediate resistance to water uptake and protection against water loss.
- After adequate light activation, they can immediately be contoured under an air-water spray at an intermediate-high speed.
Cavity Preparation Of Glass Ionomer Restorations
Glass ionomer restoration bonds to the tooth surface chemically. The following steps are involved in the cavity preparation of glass ionomer restoration:
- Removal of various enamel and dentin is the primary objective of cavity preparation.
- The affected dentin is left behind and the cave surface is smoothened.
- The tooth surface is cleaned with a slurry of pumice and water.
- The cavity is conditioned by applying 10% polyacrylic acid (GIC liquid) for 10 seconds (explained below).
- The tooth is then washed vigorously with water and air-dried for 10 seconds. Care is taken so that the dentin is not desiccated or dehydrated.
- The placement of the restoration is assisted with a matrix strip.
- The GIC is syringed into the cavity and adapted.
- Carving and removal of excess cement are done before 4 minutes (initial set).
- The final polishing is done after 24 hours under an air-water spray to prevent dehydration during the restoration.
Cavity Preparation Of Glass Ionomer Restorations Cavity Conditioning
Conditioning of the cavity treats the smear layer in two ways. The smear layer is the loosened dentinal debris at the cut surface of the dentinal tubules. Efficient adhesion of the restoration is acquired if the smear layer is removed during the cavity preparation.
Conditioning involves complete or partial removal of the smear layer to provide a clean surface for adhesion or pre-activation of the smear layer for it to be incorporated into the restoration.
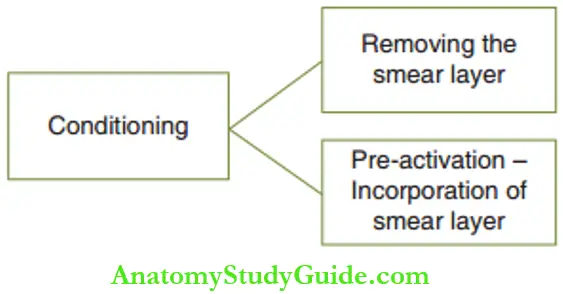
Wettability is a measure of the ease of the tooth surface to adhere to the restorative material without entrapment of air bubbles at the contact surface. The high surface energy of the GIC and the tooth surface can impair wettability.
Hence, the surface of enamel and dentin has to be pre-activated by lowering its surface energy to enhance wettability. This preactivation process incorporates the smear layer into the restoration.
Low-molecular-weight acids, such as citric acid or hydrogen peroxide, were initially used for removing the smear layer. Conditioning the cavity surfaces with the glass ionomer liquid for 10 seconds and washing the tooth surface thoroughly is the most accepted method in recent times.
Polyacrylic acid is a constituent of glass ionomer liquid. A low concentration of polyacrylic acid is an ideal mode to remove the smear layer due to the following reasons:
- An excess of the conditioner does not affect the setting reaction.
- Polyacrylic acid is a high-molecular-weight mild acid that will not demineralize the tooth surface or penetrate the tubules to initiate a pulpal reaction.
- The application of polyacrylic acid for 10 seconds will leave behind smear plugs in the dentinal tubules, which will prevent the flow of the dentinal fluid into the prepared cavity, thus keeping it dry.
The alternative method of incorporating the smear layer after pre-activation is done with mineralizing solutions such as Levine mineralizing solution or 25% tannic acid. One- to two-minute application followed by thorough washing will alter (pre-activate) the smear layer and allow its incorporation into the restoration.
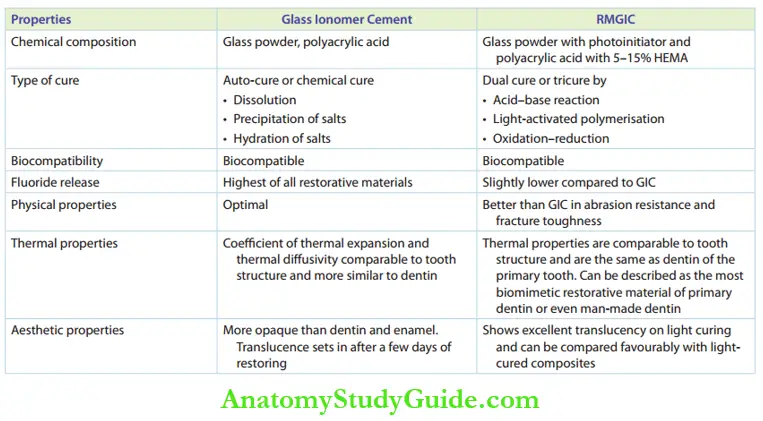
Properties Of GIC
GICs have a predominant role in pediatric operative dentistry. Their properties need elaborate mention. The properties of GICs and resin-modified GICs are compared in Table.
Features of Comparison Between Glass Ionomer Cement and Resin-Modified Glass Ionomer Cement (RMGIC)
1. Chemical Bonding To Tooth Structure
The direct chemical bonding of GIC to the tooth structure can be explained in four simple steps as depicted.
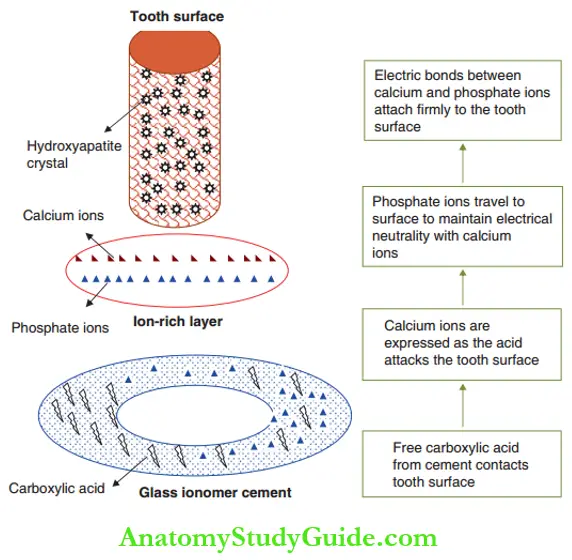
- Step 1: Acid contact: Free carboxylic acid contacts the tooth surface and reacts with hydroxyapatite crystals of enamel and dentin.
- Step 2: Expression of calcium ions:
- Calcium ions are displaced from hydroxyapatite crystals by free carboxylic acid.
- The portion of the tooth surface in contact with the cement is called the primary tooth surface.
- The layer deeper to the primary tooth surface and not in direct contact is the secondary tooth surface.
- The attack of acid on the primary tooth surface drives the calcium ions (Ca2+) to the secondary surface.
- Step 3: Migration of phosphate ions: Free anionic phosphate ions (PO43–) are released from GIC powder on reaction with a carboxylic acid. This neutrality with cationic calcium ions.
- Step 4: Formation of electrical bond: The ion-rich layer comprising electrical bonds between calcium and phosphate ions bonds firmly with the tooth structure.
Chemical Bonding To Tooth Structure Clinical Significance
Free carboxylic acid/carboxylic acid ions are required to displace calcium ions from the tooth structure. When the working time of the cement has elapsed, there will be a lesser proportion of free acids releasing fewer calcium ions.
The restoration has insufficient bond strength when this cement is applied to the cavity. This can be clinically identified as the mixed cement loses its gloss when the working time has elapsed due to the decrease in free acids.
The diffusion of calcium ions, formation of a tight bond with the ion-rich layer, and a complete adhesion of the cement with the tooth require a clean, dry tooth structure.
The ion-rich layer is firmly attached to the tooth structure and any fracture of the restoration will be between the layers of the cement (cohesive) and never at the tooth–cement interface (adhesive).
2. Fluoride Release
The attack of glass ionomer powder by carboxylic acid groups releases calcium, aluminum, Floride, phosphate, and sodium ions.
if these, fluoride ions either form insoluble complexes or stay free in the porous matrix. It implies that the free fluoride ions can move out of the matrix when there is an acidic environment.
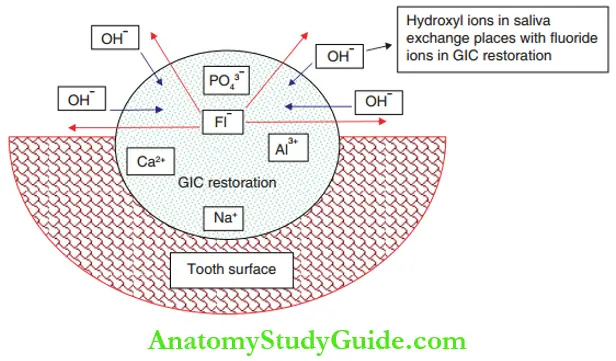
The hydroxyl ions (OH–) in the saliva have the same ionic radius, ionic charge, and valency as the fluoride ions. So, the fluoride ions exchange places with the hydroxyl ions. Fluoride exchange can take place throughout the life of the restoration, although the quantity of ionic exchange follows a pattern of variation.
The fluoride release from glass ionomer is of the order of 10 ppm, which can initiate remineralization. This accounts for the anti-cariogenicity of glass ionomers. When the fluoride concentration is high in the oral cavity like during professional application of topical fluoride, the glass ionomer restoration can take up this fluoride.
The absorbed fluoride can be released later. Hence, glass ionomer restorations can be regarded as ‘fluoride reservoirs’.
Fluoride Release Pattern with Glass Ionomer Cement

3. Biocompatibility
Glass ionomers are biocompatible. The fluoride released by glass ionomer restoration inhibits the bacterial enzyme enolase of Streptococcus mutants, the major pathogen in dental plaque.
This, bacterial plaque fails to thrive on the surface of glass ionomer restorations. Glass ionomers also exhibit a favorable soft-tissue response. Freshly mixed GIC has a pH of 0.9–1.6. The pH begins to rise in 1 hour.
The restoration does not require a liner or base as the hydroxyapatite of dentin itself can buffer the low pH. Any inflammatory reaction of the pulp usually resolves in 15 days.
4. Physical Properties
Glass ionomer materials have optimal compressive strength but poor shear resistance. They are weak and lack rigidity. They are susceptible to fracture and thus are not indicated in restorations that may be subjected to heavy occlusal loads. Glass ionomers have poor abrasion resistance.
Silver cermet materials have better abrasion resistance but the proneness to fracture of glass ionomer does not seem to reduce with this modification.
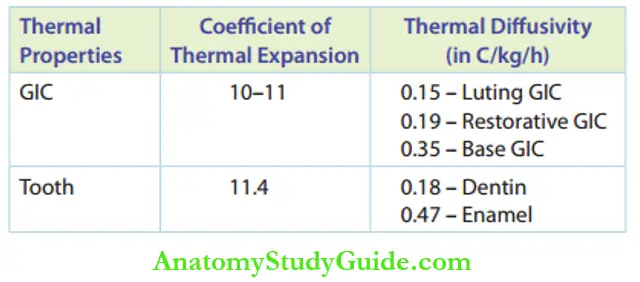
5. Thermal Properties
Thermal properties such as diffusivity and coefficient of thermal expansion of GIC are quite acceptable as they are in coherence with tooth material and more similar to dentin than to enamel.
Thermal Properties of Glass Ionomer Cement in Comparison to Tooth
6. Aesthetic Properties
The radiopacity of glass ionomers is higher than that of dentin and enamel. The set cement gains translucency in a few days of restoration after which it seems more aesthetically acceptable.
It happens by slow absorption of water or an increase in the hydration status of the set cement. Maintaining the hydration or the water content of the set cement is the key to obtaining maximum aesthetics. For the restoration to gain maximum radiolucency (aesthesis):
It has to be protected from dehydration, which can be accomplished by applying a sealant over the set cement. The final contouring of the set cement can be delayed to prevent dehydration.
Summary
1. Plastic restorative materials (glass ionomers) have significant advantages over rigid restorative materials (amalgam)
- Conservative cavity preparation – Only the carious structure is excavated.
- Involves a relatively shorter time for both cavity preparation and restoration, which respects the short attention span of children.
- Materials are tooth colored and supplied with shade specifications.
2. The various plastic materials used are as follows:
3. Indications
4. Smear layer – conditioning

Leave a Reply