Discuss briefly the uses of atropine. Write the rationale for the use of glycopyrrolate in pre-anesthetic medication.
1. As antispasmodics:
Antispasmodics or spasmolytics like atropine, dicyclomine, and propiverine relieve spasms of the gastrointestinal tract, biliary tract, ureter, and uterus. Some of them also reduce gastrointestinal motility.
- In diarrhea and dysentery—relieve colic and abdominal pain.
- In renal and biliary colic—used with morphine, atropine partly overcomes the spasm of the sphincter of Oddi.
Read And Learn More: Pharmacology Question And Answers
2. As mydriatic and cycloplegic:
Mydriasis and cycloplegia produced by atropine last for 7– 10 days. The derivatives homatropine, eucatropine, and cyclopentolate have shorter action (6– 24 hours), and tropicamide is the shortest acting (4–6 hours). Some can selectively produce mydriasis or cycloplegia.
- Diagnostic: For testing errors of refraction and fundoscopic examination of the eye.
- Therapeutic: To provide rest to the iris in iritis, iridocyclitis, and keratitis after iridectomy.
- Mydriatics are used alternately with miotics to break the adhesions (synechiae) between the iris and lens in uveitis and iritis.
3. As pre-anesthetic medication: Given 30 minutes IM before anesthesia, atropine reduces salivary and respiratory secretions but glycopyrrolate is preferred for this use because:
Glycopyrrolate an antisialogogue reduces salivary secretions and this will prevent the development of laryngospasm and aspiration pneumonia.
- Prevents bradycardia during surgery.
- Has bronchodilator action—is of additional value.
- It is a quaternary ammonium compound, does not cross the BBB → no effects on CNS

4. In poisoning:
- Organophosphorus poisoning: Atropine is life-saving in OP poisoning.
- Mushroom poisoning: Atropine is used (Inocybe family mushrooms).
- Curare poisoning: Atropine is used with neostigmine (to block muscarinic effects).
5. Bronchial asthma and COPD: Atropine thickens the bronchial secretions and interferes with the movement of cilia, thus leading to the formation of mucus plugs in bronchial asthma and COPD. However, the derivatives are useful.
- Ipratropium and tiotropium are bronchodilators (block M3 receptors)
- Do not affect mucociliary activity.
- Given as inhalation, act only on the airways and have no significant systemic effects because they are poorly absorbed.
- Tiotropium is longer-acting (OD):
6. Peptic ulcer: Pirenzepine and telenzepine are selective M1 blockers—selectively inhibit gastric secretions. Pirenzepine also does not cross the BBB, hence has no CNS effects.
7. Parkinsonism: Benztropine, benzhexol, and biperiden are used in drug-induced Parkinsonism due to their anticholinergic effects. They control tremors and rigidity

8. In cardiovascular disorders: Atropine produces tachycardia—hence useful in patients with bradycardia or partial heart block due to excessive vagal tone.
9. Motion sickness: Hyoscine given 30 minutes before the journey prevents traveling sickness.
- The exact mode of action is not known but scopolamine acts as a vestibular sedative by blocking the muscarinic receptors.
- Transdermal hyoscine patches are applied behind the ear for prolonged action.
10. Urinary disorders: As muscarinic receptors are present in the epithelial cells lining the urinary tract, atropine and its vasicoselective derivatives are used in urinary disorders like in overactive bladder and urinary incontinence.
- Darifenacin, solifenacin, tolterodine, fesoterodine, oxybutynin, and trospium are more M3 selective in the bladder, and therefore anticholinergic side effects are
negligible. - They reduce urinary urgency and frequency, relieve bladder spasms and improve bladder capacity and reduce involuntary voiding in urinary disorders and following urologic surgeries.
- Also tried in nocturnal enuresis in children.
- Oxybutynin can be given orally or instilled into the bladder cavity or applied transdermally.
11. Labor: Hyoscine can also be used during labor to produce sedation and amnesia.
Drug interactions: Other drugs that have anticholinergic properties like antihistaminics,
phenothiazines and tricyclic antidepressants add to the anticholinergic side effects.

Ganglion blockers: Trimethaphan and hexamethonium block the autonomic ganglia. They produce a wide range of adverse effects and are therefore not used. Trimethaphan is rarely used IV to produce transient hypotension.
Skeletal Muscle Relaxants
Skeletal muscle relaxants (SMRs) are drugs that reduce the muscle tone either by acting peripherally at the neuromuscular junction or centrally in the cerebrospinal axis or directly on the contractile mechanism.
Skeletal muscle relaxant Classification:

Skeletal Muscle Relaxants Peripherally Acting:
Competitive blockers/Neuromuscular Blockers (NMB)
Tubocurarine: Curare was used by the South American Indians as arrow poison for hunting wild animals. d-tubocurarine (dTC) is an alkaloid obtained from the plant Chondrodendron tomentosum. Several synthetic agents have been developed.
Mechanism of action:
- Nondepolarizing blockers bind to Nm nicotinic receptors on the motor end plate and competitively block the actions of acetylcholine.
- As these compounds slowly dissociate from the receptors and transmission is gradually restored → the action of dTc is reversible.
- Increasing the concentration of the agonist, i.e. acetylcholine at the NMJ by anticholinesterases like neostigmine overcomes the blockade.

Skeletal Muscle Relaxants Pharmacological Actions:
1. Skeletal muscle:
- On parenteral administration, tubocurarine initially causes muscular weakness followed by flaccid paralysis. Order of paralysis (see mind map). Consciousness is not affected throughout.
- Recovery occurs in the reverse order, i.e. the diaphragm is the first to recover. The effect lasts for 30–60 minutes.
2. Autonomic ganglia: In high doses, dTC blocks autonomic ganglia and adrenal medulla resulting in hypotension.
3. Histamine release: Tubocurarine can cause histamine release from the mast cells leading to hypotension, bronchospasm, and increased tracheobronchial and gastric secretions.
Skeletal Muscle Relaxants Adverse Reactions:
- Respiratory paralysis and prolonged apnea—artificial ventilation needed. Neostigmine
reverses skeletal muscle paralysis and antihistamines counter the effects of histamine - Hypotension is due to ganglion blockade and histamine release.
- Flushing and bronchospasm due to histamine release by tubocurarine; this is not seen with newer agents.
Mind Map Of Neuromuscular Blockers (Nmbs):

Treatment Of Toxicity/Curare Poisoning
- Antidote: Neostigmine and edrophonium reverse skeletal muscle paralysis and are the antidotes. They may be also used to reverse the neuromuscular (NM) blockade after the surgical procedure is completed.
- Antihistamines: Antihistamines are given to counter the effects of histamine.
- Sugammadex: Sugammadex is the antidote in the overdosage of rocuronium and vecuronium. It binds and chelates them and quickly reverses their effects. The complex is excreted in the urine. It can also chelate other NMBs like pancuronium to some extent.
Sugammadex → Binds to and chelates rocuronium, vecuronium → Complex excreted in urine
Synthetic competitive blockers include:
Pancuronium, atracurium, vecuronium, doxacurium, mivacurium, pipecuronium, rapacuronium, rocuronium.
Their advantages over DTC are:
- Less histamine release
- Do not block autonomic ganglia, hence causing less hypotension
- Spontaneous recovery with most of these drugs
- Some are more potent than dTC.
1. Rapacuronium and rocuronium:
Rapacuronium and rocuronium have a rapid onset of action → Can be used as alternatives to succinylcholine for muscle relaxation.
2. Atracurium:
Atracurium can be safely used in patients with renal impairment because it is degraded spontaneously by plasma esterases by Hofmann elimination and does not depend on the kidney for excretion.
Toxicity Disadvantage:
A metabolite of atracurium (laudanosine) can cause seizures.
Cisatracurium: Toxicity Advantages:
- Forms lesser laudanosine—less risk of seizures.
- Lesser histamine release when compared to atracurium.
- Also metabolized by Hofmann elimination can be given in renal dysfunction.
Therefore cisatracurium is now preferred over atracurium.
3. Mivacurium: Mivacurium is a short-acting NMB with a slow onset of action.
4. Tubocurarine: Tubocurarine causes histamine release, ganglion blockade, and its muscle relaxant effect needs to be reversed with drugs → hence, not used now. Synthetic compounds are used.
Depolarizing Blockers
Succinylcholine (SCh, suxamethonium) is a quaternary ammonium compound and structure resembles two molecules of acetylcholine joined together.
Depolarizing Blockers Mechanism of action: Like ACh, SCh stimulates the NM nicotinic receptors and depolarizes the skeletal muscle membrane.
However, unlike ACh which is destroyed in milliseconds, SCh is destroyed very slowly by pseudocholinesterase (in about 5 minutes). This continued presence of the drug causes persistent depolarization resulting in flaccid paralysis (phase I block).
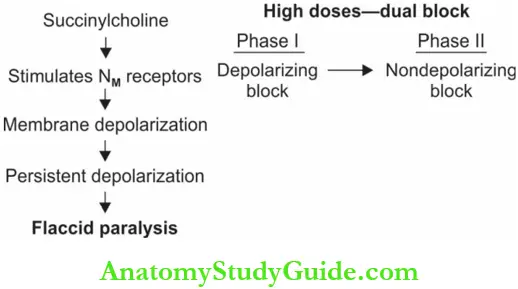
In high doses → SCh produces a dual block—an initial depolarizing block followed by a non-depolarizing block. Though the membrane gets slowly repolarized after phase I, it cannot be depolarized again. The mechanism is not clearly known.
Depolarizing Blockers Pharmacological Actions:

- Followed by—stimulation of sympathetic ganglia—↑ BP, ↑ HR
- Higher doses—cardiac arrhythmias. SCh can also cause histamine release.
Depolarizing Blockers Pharmacokinetics:
- Succinylcholine is rapidly hydrolyzed by pseudocholinesterase (in 5minutes).
- Some people (1 in 2,000) have an abnormal (atypical) pseudocholinesterase enzyme, which takes a longer time to metabolize SCh, and even the usual dose results in prolonged apnea and paralysis for several hours.
- Artificial ventilation and fresh blood transfusion are needed to supply pseudocholinesterase.

1. Postoperative muscle pain: Due to damage to muscle fibers during initial fasciculations.
2. Hyperkalemia: Sudden release of K+ from intracellular sites due to fasciculations → cardiac arrest in patients with CCF, burns, nerve injuries, and neuromuscular disease.
3. Cardiac arrhythmias: SCh stimulates NN nicotinic receptors in the ganglia and cardiac muscarinic receptors → arrhythmias.
4. Malignant hyperthermia: Malignant hyperthermia is a rare genetically determined condition where there is a sudden increase in the body temperature and severe muscle spasm due to the release of intracellular Ca++ from the sarcoplasmic reticulum.
- Metabolic acidosis and tachycardia may be present. Drugs like halothane, isoflurane, sevoflurane, and succinylcholine, or a combination of them can trigger the process which can be fatal.
- Intravenous dantrolene (1 mg/kg repeated if required) is life-saving in malignant hyperthermia. Oxygen inhalation, treatment of acidosis, and immediate cooling of the body also help.
5. Masseter muscle rigidity or soreness: Seen in genetically predisposed patients → difficulty in intubation.
6. Others:
![]()
- Intraocular pressure: May also be increased.
Tubocurarine and Succinylcholine:

Uses of SMRs:
Inappropriate use of peripherally acting SMRs can be fatal. Hence, they should be given only by qualified anesthetists or adequately trained doctors:
- Adjuvant to anesthesia: Adequate muscle relaxation is essential during surgeries. SMRs are used as adjuvants to general anesthesia.
- In minor procedures: SMRs useful in laryngoscopy, bronchoscopy, esophagoscopy, tracheal intubation and in orthopedic procedures like reduction of fractures and dislocations.
- In electroconvulsive therapy: SMRs protect the patient from convulsions and trauma during
ECT. - In spastic disorders: SMRs overcome the spasm of tetanus and athetosis.
- In status epilepticus: When convulsions cannot be controlled by anticonvulsants alone, an NMB is used to control the muscular component of convulsions.
- In patients on ventilator: To reduce the resistance of the chest wall and increase thoracic compliance and to facilitate artificial ventilation, NMBs are used in intensive care units.

Salient features of skeletal muscle relaxants are given in Table
Salient features of skeletal muscle relaxants:

Other Drugs Acting At NMJ
Botulinum toxin:
- Botulinum toxin produced by Clostridium botulinum inhibits the release of acetylcholine at the cholinergic synapses resulting in flaccid paralysis of skeletal muscles.
- Botulinum toxin is useful (local injection) in the treatment of dystonias including sports or writer’s cramps, muscle spasms, tremors, cerebral palsy, and in rigidity seen in extrapyramidal disorders. It is commonly used to relieve blepharospasm.
- Botulinum toxin is also gaining popularity in cosmetic therapy for the removal of facial lines and wrinkles by local injection.
Directly Acting Muscle Relaxants
Dantrolene:
Dantrolene is a phenytoin analog that acts directly on skeletal muscle.
Directly Acting Muscle Relaxants Mechanism of action:
- Dantrolene directly affects the skeletal muscle contractile mechanism.
- It binds to be ryanodine receptor (RyR1) and blocks the opening of the ryanodine channel.
- It prevents calcium release from the sarcoplasmic reticulum and thus inhibits muscle contraction. Dantrolene is effective orally
Directly Acting Muscle Relaxants Adverse effects:
Including drowsiness, dizziness, fatigue, diarrhea, muscle weakness, and rarely hepatotoxicity. Liver function tests should be done to look for hepatotoxicity.
Directly Acting Muscle Relaxants Uses:
- Spastic disorders like hemiplegia and paraplegia, multiple sclerosis, and spinal cord injury.
- Drug of choice in malignant hyperthermia. Dantrolene prevents the release of Ca++ from
the sarcoplasmic reticulum and relieves muscle spasms in malignant hyperthermia. - Useful in malignant neuroleptic syndrome.
Directly Acting Muscle Relaxants Quinine:
The antimalarial quinine is a sodium channel blocker, it reduces the excitability of the motor end plate. Quinine is used in myotonia congenita and in nocturnal muscle cramps.
Mechanism of action:

Centrally Acting Muscle Relaxants
Drugs in the category are:
- GABAA agonists Benzodiazepines like diazepam
- GABAB agonist Baclofen
- Central α2 agonist Tizanidine
Centrally Acting Muscle RelaxantsOthers:
Mephenesin, carisoprodol, chlorzoxazone, chlormezanone, methocarbamol, thiocolchicoside, riluzole, gabapentin, progabide.
Centrally Acting Muscle Relaxants Mechanism of action:
- Spinal polysynaptic reflexes maintain muscle tone.
- By depressing these spinal reflexes, centrally acting muscle relaxants reduce muscle tone without loss of consciousness.
- They also have sedative properties.
Centrally Acting Muscle Relaxants Baclofen:
- Baclofen is an analog of the inhibitory neurotransmitter GABA.
- It is a GABAB agonist-depresses the monosynaptic and polysynaptic reflexes in the spinal cord and relieves painful spasms.
- May improve bladder and bowel functions in patients with spinal lesions. Normal tendon reflexes are not affected.
- Baclofen also inhibits the release of substance P which could contribute to pain relief. Given orally it is rapidly and completely absorbed.
- Dose: 10–50 mg BD.

Baclofen Side effects:
- Mild drowsiness, weakness, and ataxia.
- It should be gradually withdrawn after prolonged use because abrupt withdrawal can cause anxiety, palpitations, and hallucinations.
Baclofen Uses:
- Muscle spasms in patients with spinal lesions
- Severe low back pain
- Alcohol deaddiction → reduces craving
Centrally Acting Muscle Relaxants Tizanidine:
- Tizanidine a congener of clonidine is a central a2 -agonist like clonidine. It increases the presynaptic inhibition of motor neurons and reduces muscle spasms.
- The muscle relaxant effect is seen in lower doses (2 mg TDS) and the cardiovascular effects are not significant at such doses.
- Adverse effects include drowsiness, weakness, hypotension, and dry mouth.
Riluzole, gabapentin, and progabide inhibit glutamate release in the CNS:
- They are well tolerated with minor adverse effects like nausea and diarrhea.
- Thiocolchicoside related to colchicine acts as a GABA mimetic and is used with NSAIDs for painful muscle spasms.
Uses Of Centrally Acting Muscle Relaxants
- Musculoskeletal disorders like muscle strains, sprains, myalgias, cervical root syndromes, herniated disc syndromes, low backache, dislocations, arthritis, fibrositis, and bursitis all cause painful muscle spasms. Muscle relaxants are used with analgesics in these conditions.
- Spastic neurological disorders like cerebral palsy, multiple sclerosis, poliomyelitis, hemiplegia, and quadriplegia are treated with diazepam, baclofen or tizanidine.
- Orthopedic procedures like fracture reduction may be done after administering diazepam.
- ECT: Diazepam is given with peripherally acting SMRs.
- Tetanus: Diazepam is given IV

Drugs Used In The Treatment Of Local Muscle Spasms:
Cyclobenzaprine, metaxalone, carisoprodol, chlorzoxazone, meprobamate, and methocarbamol are used for the treatment of local muscle spasms which may result from injury or strain.
They have the following common features:
- All these drugs act by depressing spinal polysynaptic reflexes.
- ADR—drowsiness and dizziness.
- Cyclobenzaprine causes dryness of the mouth and anticholinergic effects.
- Many of them are available in combination with NSAIDs.
- NSAIDs effectively relieve muscle spasms which could be due to inflammation.
Drug Interactions With Smrs:
- General anesthetics → ↑ ↑ SMR action
- Anticholinesterases like neostigmine reverse the action of NMBs.
- Aminoglycosides and calcium channel blockers → potentiate SMR action.
- Succinylcholine + halothane → increased risk of malignant hyperthermia.
Adrenergic System
Adrenergic System of Functions:
The prime function of the adrenergic or sympathetic nervous system is to help the human being to adjust to stress and prepare the body for fight or flight reactions. For example, if he is being chased by a tiger.
When exposed to sudden stress:
- Heart rate and stroke volume increase → ↑ ↑ cardiac output (more O2 needed).
- Blood is shifted from the skin, gut, kidney, and glands (vasoconstriction in these areas) to the heart, skeletal muscles, brain, and lungs (vasodilatation in these organs) because these organs need more blood during stress.
- Pupils dilated → To see better
- Bronchodilatation → Better oxygenation
- Sweating is increased → To cool the body.
- Blood glucose increases → By glycogenolysis

Adrenergic System of Neurotransmitters:
- Adrenergic neurotransmitters: Noradrenaline (NA, norepinephrine) and dopamine (DA).
- Adrenaline (epinephrine) is the major hormone secreted by the adrenal medulla (85% adrenaline and 15% NA).
Synthesis, storage, and release of catecholamines:
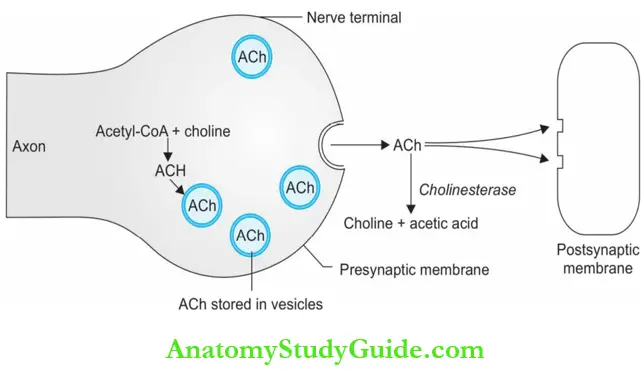
The sympathetic postganglionic nerve fibers that synthesize, store, and release NA are called adrenergic.
- Noradrenaline is stored in small vesicles in the adrenergic nerve terminals.
- In response to nerve impulses, NA is released into the synaptic cleft by exocytosis.
- This NA binds to adrenergic receptors present on the postsynaptic membrane to produce the response. A small portion of NA is metabolized by the enzyme COMT.
- A large portion (nearly 80%) is taken back into the nerve terminals by an energy-dependent active transport process termed uptake 1 with the help of an amine pump norepinephrine transporter (NET) thus terminating the action of NA.
- Of this, a fraction is metabolized by MAO present in mitochondria, and the remaining NA is then transferred to the storage vesicles by another amine pump called vesicular monoamine transporter 2 (VMAT2).
- Some part of NA released into the synaptic cleft penetrates into the effector cells and is known as uptake 2 with the help of another amine pump [called extraneuronal amine transporter (ENT)].

Adrenergic Receptors:
- Ahlquist classified adrenergic receptors into 2 types:
- α and β which are further classified into subtypes given in below. Both a and b-adrenergic receptors are GPCRs.
- Stimulation of a-receptors → activates phospholipase C → acts through the generation of second messengers IP3 and DAG and increases intracellular calcium.
- Stimulation of b-receptors → activates enzyme adenylyl cyclase → ↑ ↑ intracellular cyclic AMP levels which acts through intracellular proteins → response.
- Stimulation of α-receptors mainly produces excitatory effects (exception-GIT); stimulation causes mainly inhibitory effects (exception-heart).
- Stimulation of presynaptic a2 receptors inhibits the further release of NA as they exert negative feedback on NA release.
- There are five subtypes of DA receptors (D1 – D5) which are all GPCRs

Characteristics of adrenergic receptors:

Adrenergic Drugs
Adrenergic drugs or sympathomimetics are drugs whose actions mimic that of sympathetic stimulation. Adrenergic drugs may be classified in 2 different ways as below:
Adrenergic Drugs Classification:
- Chemical classification based on the presence/absence of catechol nucleus:
- Catecholamines: Noradrenaline, adrenaline, dopamine
- Synthetic: Isoprenaline, dobutamine, dopexamine, dipivefrine
- Noncatecholamines: Ephedrine, amphetamine
- Therapeutic/clinical classification:
- Vasopressors: Noradrenaline, dopamine, phenylephrine, methoxamine, mephentermine, metaraminol
- Cardiac stimulants: Adrenaline, dopamine, dopexamine, dobutamine, fenoldopam, isoprenaline, ephedrine
- CNS stimulants: Amphetamine, dexamphetamine, ephedrine
- Bronchodilators: Adrenaline, isoprenaline, salbutamol, terbutaline, salmeterol, perbuterol, fenoterol, formoterol
- Nasal decongestants: Ephedrine, pseudoephedrine, phenylpropanolamine, phenylephrine, oxymetazoline, xylometazoline, naphazoline
- Uterine relaxants: Salbutamol, terbutaline, isoxsuprine, ritodrine
- Appetite suppressants (anorectics): Fenfluramine
Leave a Reply