Cellular Responses To Stress And Toxic Insults
Question 1. Define atrophy with examples.
Answer:
Atrophy
Is defined as a reduction in the size of an organ or tissue due to a decrease in cell size and number
Examples
- Physiologic atrophy: Seen in a reduction in the size of the uterus after childbirth.
- Pathologic atrophy: Seen in disuse atrophy (disuse of any organ), denervation atrophy (loss of nerve supply), diminished blood supply, inadequate nutrition, loss of endocrine stimulation, and pressure.
Read and Learn More Preparatory Manual of Pathology Question and Answers
Question 2. Define metaplasia with examples.
Answer:
Metaplasia
- Reversible change in which one differentiated cell type (epithelial or mesenchymal) is replaced by another cell type.
Examples
- Columnar to squamous: Most common epithelial metaplasia.
- Barrett esophagus: Metaplasia from squamous to columnar type.
- Connective tissue metaplasia: Formation of cartilage, bone, or adipose tissue (mesenchymal tissues) in tissues that normally do not contain these elements.
Question 3. Define necrosis. Discuss in detail with examples the different types of necrosis.
Answer:
1. Necrosis
- Occurs due to the denaturation of intracellular proteins and enzymatic digestion of lethally injured cells.
- Due to damage to cellular plasma membranes, their protein contents leak out, which may elicit an inflammatory reaction.
Morphology
- Necrotic cells show increased eosinophilia and have a glassy homogeneous appearance.
- Myelin figures: Large, whorled phospholipid masses, which represent damaged cell membranes.
Electron microscopy: Shows marked dilation of mitochondria with the appearance of large amorphous densities.
Nuclear changes
- Karyolysis: Basophilia of chromatin may fade.
- Pyknosis: Nuclear shrinkage and increased basophilia.
- Karyorrhexis: Pyknotic nucleus undergoes fragmentation and loss.
2. Patterns of Tissue Necrosis with Examples
1. Coagulative necrosis
- Cause: Ischemia caused by an obstruction in a vessel
- Site: Can be seen in all organs except the brain
- The architecture of dead tissues is preserved for a few days
- Results in the formation of eosinophilic, anucleate cells, which are removed by phagocytosis
- Infarct: Localized area of coagulative necrosis.
2. Liquefactive necrosis
- Characterized by digestion of the dead cells, which results in the transformation of the tissue into a liquid viscous mass
- Seen in bacterial or fungal infections and affects most commonly, the central nervous system.
3. . Gangrenous necrosis
- For example, the lower limb, which has lost its blood supply and has undergone necrosis
- Represents a coagulative pattern of necrosis
- Wet gangrene: There occurs Superimposed bacterial infection, resulting in liquefactive necrosis.
4. Caseous necrosis
- Caseous (cheese-like) area: Friable white appearance of necrosis
- Microscopy: Lysed cells and amorphous granular debris with inflammatory cells, and a resultant granuloma formation.
5. Fat necrosis
- Refers to the focal area of fat destruction in the pancreas and peritoneum
- Occurs due to the release of pancreatic lipases from the pancreas in acute pancreatitis
- Released lipases split triglyceride esters within fat cells, which combine with calcium to produce chalky-white areas (fat saponification).
6. Fibrinoid necrosis
- Seen in immune reactions involving the blood vessels Cellular Responses to Stress and Toxic Insults 5
- Occurs due to deposition of antigen-antibody complexes and fibrin (that has leaked out of vessel wall) in the vessel wall
- On H and E stain: The resultant deposit gives a bright pink and amorphous appearance, called “fibrinoid” (fibrin-like).
Question 4. Discuss in detail free radical injury, its mechanism, and its pathological effects.
Answer:
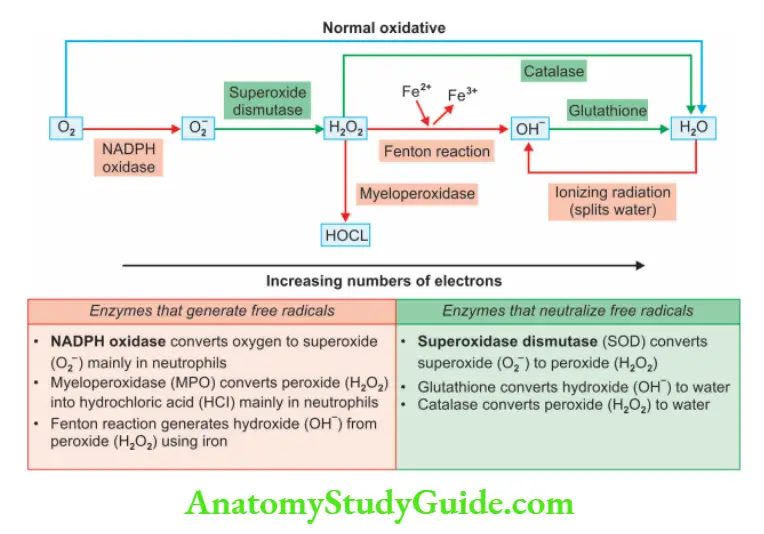
What are free radicals?
- Free radicals have a single unpaired electron in their outer orbit
- Reactive oxygen species (ROS)—implies oxygen-derived free radicals
- Oxidative stress: Characterized by an excess of free radicals
Free radical injury is divided into 3 phases
1. Generation of free radicals
- Reduction–oxidation reaction occurring during normal metabolic processes produces:
Superoxide anion (O2one electron), hydrogen peroxide (H2O2, two electrons), and hydroxyl ions (OH, three electrons) - Absorption of radiant energy: For example, ultraviolet light and X-rays
Produces OH and hydrogen (H) free radicals - Activated leucocytes in inflammation produce O2
- Transition metals such as iron and copper as seen in the Fenton reaction
H2O2 + Fe2+→Fe3+ + OH + OH– - Nitric oxide (NO): Converted to highly reactive peroxynitrite anion
(ONOO–),NO2 and NO–3
2. Removal of free radicals
- Antioxidants: Blocks free radical formation or inactivate free radicals, for example, vitamins E and A, ascorbic acid, and glutathione
- Enzymes that act as free radical-scavenging systems:
- Catalase (in peroxisomes)—decomposes H2O2 (2H2O2 → O2 + 2H2O)
- Superoxidase dismutases (SODs)—manganese SOD (in mitochondria) and copper zinc-SOD (in the cytosol)—convert O–2 to H2O2
- Glutathione peroxidase (mitochondria and cytosol)—leads to free radical breakdown (H2O2 + 2GSH ® GSSG [oxidized glutathione] + 2H2O) or (2OH + 2GSH ® GSSG + 2H 2O).
3. Pathological effects of free radical injury
- Lipid peroxidation in membranes, with resultant membrane damage
- Oxidative modification of proteins, with resultant protein misfolding and breakdown
- Lesions in DNA, with resultant DNA damage and mutations.
Question 5. Define apoptosis. Discuss in detail the morphological changes, mechanisms, and examples of apoptosis.
Answer:
Definition: Programmed cell death in which the cells destined to die, activate intrinsic enzymes that degrade the cell’s own nuclear DNA and nuclear and cytoplasmic proteins.
Morphology
- Cell shrinkage (cell swelling is an early feature in other forms of cell injury)
- Chromatin condensation
- Formation of cytoplasmic blebs and apoptotic bodies
- Phagocytosis of apoptotic cells or cell bodies
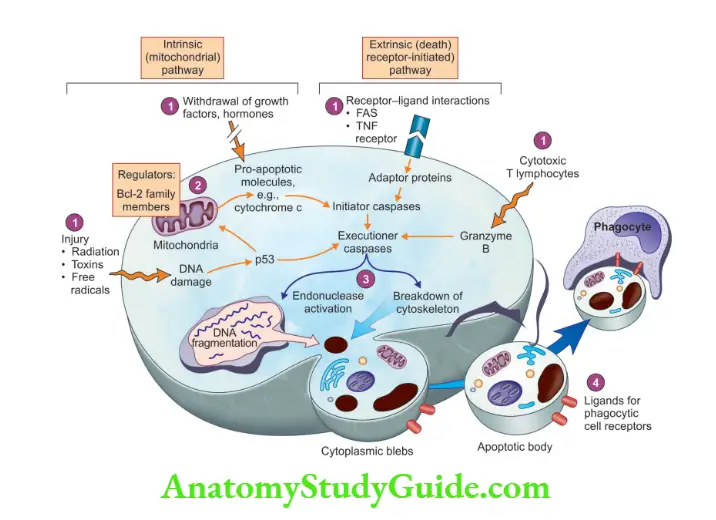
Mechanism of apoptosis: Apoptosis results from the activation of enzymes called caspases.
Two Phases
1. Initiation phase
1. Intrinsic (mitochondrial) pathway of apoptosis:
- Growth factors stimulate the release of anti-apoptotic proteins, which include Bcl-2, Bcl-x, and Mcl-1
- In the absence of growth factors or when there is DNA damage, there occurs activation of the BAX/BAK channel (as BCL-2 is antagonized), resulting in leakage of mitochondrial proteins cytochrome c
- Cytochrome c (in the cytosol) binds to the protein called APAF-1 (apoptosis activating factor-1), which forms the apoptosome
- This complex is able to bind caspase-9 (critical initiator caspase of the mitochondrial pathway)
2. Extrinsic (death receptor-initiated) pathway of apoptosis:
- Initiated by the engagement of plasma membrane death receptors, which are present in a variety of cells
- Best known death receptors are type 1 TNF receptor (TNFR1) and a related protein called Fas (CD95)
- The ligand for Fas is called Fas ligand (FasL)
- FasL is expressed on T-cells that recognize self-antigens
- When FasL binds to Fas, three or more molecules of Fas are brought together, resulting in the formation of FADD (Fas-associated death domain)
- FADD that is attached to the death receptors binds with an inactive form of caspase-8 (caspase-10 in humans), via a death domain
- The inactive form of caspase-8 and caspase-10 is brought into an active form
- Extrinsic pathways can be inhibited by a protein called FLIP.
2. Execution phase
A mitochondrial pathway leads to the activation of the initiator caspase-9
- A mitochondrial pathway leads to the activation of the initiator caspase-9
- Death receptor pathway leads to activation of initiator caspases-8 and -10
- Both pathways lead to the activation of executioner caspases, caspase-3 and -6
- These enzymes cleave DNA into nucleosome-sized pieces
Examples of apoptosis in health and disease
- Growth factor deprivation results in cell death by apoptosis
- DNA Damage: p53 stimulates pro-apoptotic proteins BAX and BAK with resultant apoptotic cell death
- Protein misfolding:
- Chaperones/heat shock proteins (hsp70) in the endoplasmic reticulum (ER) result in the proper folding of newly synthesized proteins
- Unfolded protein response (UPR): Due to inherited mutations or stress, unfolded or misfolded proteins accumulate in the endoplasmic reticulum.
ER stress
- Mechanism to handle unfolded protein response
- Due to unfolded protein response, there occurs an increased production of chaperons
- Chaperons reduce the load of misfolded proteins
- If the above adaptation fails, cells activate caspases and induce apoptosis.
Question 6. List the diseases caused by abnormal protein accumulation or misfolding of proteins.
Answer:
Examples of the diseases caused by misfolding of proteins:
Cystic fibrosis, familial hypercholesterolemia, Tay-Sachs disease, Alpha-1-antitrypsin deficiency, Creutzfeldt-Jakob disease, Alzheimer’s disease, Huntington’s disease, Parkinson’s disease, Type-II diabetes.
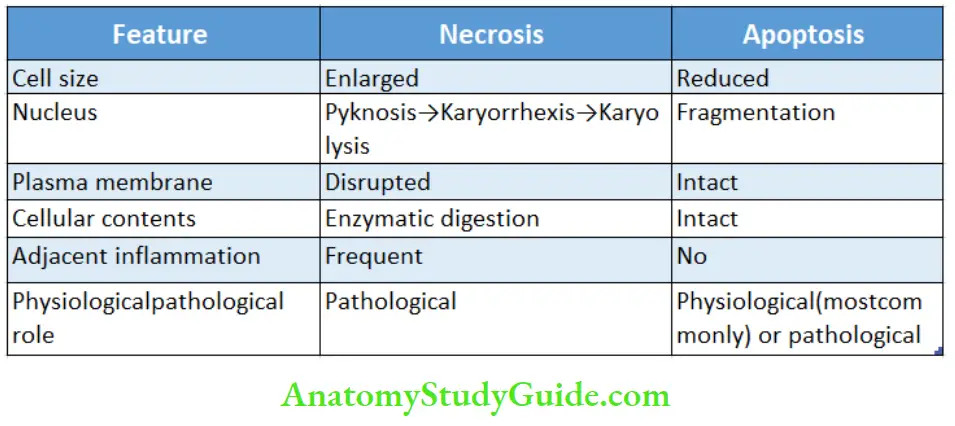
Question 7. Enumerate the differences between necrosis and apoptosis.
Answer:
Question 8. Write a note on necroptosis along with examples.
Answer:
Necroptosis
- Characterized by necrosis and apoptosis
- Morphological features resemble necrosis and mechanistically resemble apoptosis, hence called programmed necrosis
- Triggered by TNF attaching to its receptor TNFR1
- This mechanism is caspase-independent but is dependent on signaling by the RIP1– RIP3 complex, resulting in cellular swelling and membrane damage as occurs in necrosis
Necroptosis is associated with cell death in:
- Steatohepatitis (hepatocytic injury)
- Acute pancreatitis
- Reperfusion injury
- Neurodegenerative diseases such as Parkinson’s disease
- In host defense against certain viruses (for example cytomegalovirus).
Question 9. Write a note on autophagy.
Answer:
Autophagy
- Process in which a cell eats its own contents and triggers cell death
- Occurs in a nutrition-deprived cell, which survives by cannibalizing itself
- The cellular organelles are now sequestrated into the cytoplasmic autophagic vacuoles (autophagosomes)
- Autophagosomes fuse with a lysosome to form an autophagolysosome
- Cellular components are digested by lysosomal enzymes
- Regulated by a defined set of “autophagy-related genes” known as Atgs.
Question 10. Classify pigments and write a note on Lipofuscin.
Answer:
Pigments
1. Exogenous pigments:
- Carbon (coal dust)
- Tattooing
2. Endogenous pigments:
- Lipofuscin
- Melanin
- Homogentisic acid
- Hemosiderin
Lipofuscin
- Lipochrome or wear-and-tear pigment
- Its presence indicates free radical injury
- Appears as yellow-brown, finely granular cytoplasmic, peri-nuclear pigment
- Seen in the liver and heart of aging patients or severely malnourished patients.
Question 11. Write a short note on pathological calcification.
Answer:
Pathological calcification: Characterized by abnormal tissue deposition of calcium salts.
Two types
1. Dystrophic calcification:
- Deposition of calcium occurs locally in dying tissues
- Serum calcium levels are normal
- For example, seen in areas of necrosis, atheromas, aging or damaged heart valves, and tuberculous lymph node
2. Metastatic calcification:
- Deposition of calcium salts in normal tissues
- Seen in patients with hypercalcemia
- Sites—interstitial tissues of the gastric mucosa, kidneys, lungs, systemic arteries, and pulmonary veins
Causes of hypercalcemia
- Hyperparathyroidism: Increased secretion of parathyroid hormone (PTH) with subsequent bone resorption
- Resorption of bone tissue due to multiple myeloma or metastasis from breast cancer
- Vitamin D-related disorders: Intoxication, sarcoidosis
- Renal failure: Retention of phosphate, leading to secondary hyperparathyroidism.
Leave a Reply