The Cardiovascular System
Table of Contents
Anatomy
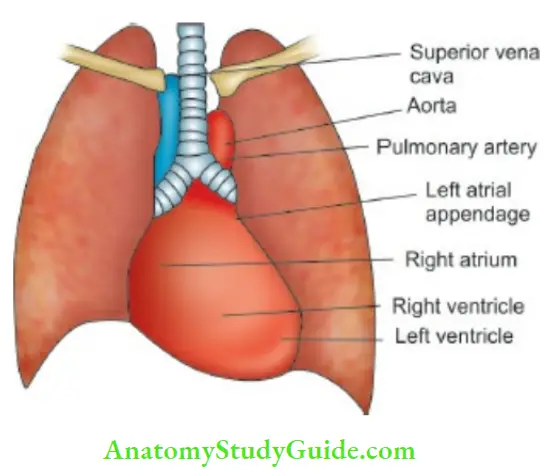
The heart is located in the middle and left side of the hemothorax. It comprises two muscular pumps working in series, covered in a serous sac (pericardium) which allows free movements with each heartbeat and respiration.
The right heart (right atrium and ventricle) pumps the blood returning from systemic veins (inferior and superior vena cavae) into the pulmonary circulation at a relatively low pressure. The left heart (left atrium and ventricle) receives oxygenated blood from the lungs and pumps it throughout the body through aorta and systemic arteries at relatively higher pressures.
Read and Learn More Pediatric Clinical Methods Notes
The heart muscle (myocardium) is thicker in the ventricles than the atria and in the left heart than the right heart in order to generate higher pressures. However, in term newborn babies, there is physiologic right ventricular preponderance.
A cardiac silhouette as seen on a PA skiagram of the chest is illustrated The atrioventricular valves (tricuspid on the right side and mitral on the left) separate the atria from the ventricles. They are attached to the papillary muscles in the myocardium of the ventricles by chordae tendineae which prevent them from prolapsing into the atria when the ventricle contracts.
The pulmonary valve on the right side of the heart and the aortic valve on the left, separate the ventricles from the pulmonary and systemic arterial systems, respectively. Each of these valves has three cusps and is called as semilunar valve because they are half-moonshaped. Cardiac contractions are coordinated by a specialized neural mechanism.
The cells in the sinoatrial node act as cardiac pacemaker. The further spread of cardiac impulse ensures that atrial contraction is completed before ventricular contraction (systole) begins. At the end of systole, the atrioventricular valves open, allowing blood to flow from the atria to refill the ventricles (diastole).
History
Ask for presenting symptoms in a lucid and chronological order. Identify the age at the onset of congestive heart failure to decide whether the patient is having congenital or acquired heart disease. In infants rapid breathing, easy fatigability while feeding, inability to suck vigorously and continuously, failure to thrive and puffiness are recognized clinical features of CHF.
In an older child, assess and grade the degree of dyspnea. Grade I dyspneic while climbing stairs or running for a short distance; Grade II dyspneic while performing daily routine activity; Grade III dyspneic following below-average activity, like going to the bathroom or walking from one room to the other; and Grade IV when child is dyspneic at rest and is orthopneic.
Older children may give a history of palpitations (unpleasant sensation of beating of the heart) while in younger children an observant mother may give a history of visible precordial pulsations and bulging of the precordium.
Ask history of and age at the onset of cyanosis, cyanotic spells, and squatting or assuming the knee-chest position. It is characterized by bouts of excessive crying, worsening of cyanosis, rapid breathing and adoption of typical posture with flexion of hips and elbows to increase systemic resistance and shunt more blood towards the lungs by reducing right-to-left shunt.
Stokes-Adams attacks may occur due to episodes of ventricular asystole occurring commonly in complete heart block causing sudden syncope which resolves spontaneously within a minute or so. The child collapses and suddenly becomes pale or white and then cyanosed; and as recovery takes place reactive hyperemia causes marked flushing.
Chest pain due to myocardial ischemia is rare in children but can occur due to pericarditis, severe aortic stenosis or regurgitation, mitral valve prolapse, anomalous origin of left coronary artery, complex congenital heart disease, dilated cardiomyopathy, and Kawasaki disease.
Chest pain due to pericarditis is precordial or retrosternal in location, radiates to the back or left shoulder, is aggravated in supine position, and is relieved by sitting and leaning forward. Ask for history of arthritis and migratory arthralgias, chorea, and syncope (due to arrhythmia).
Chorea is more common in adolescent girls. Look for purposeless jerky movements of limbs and twitchings of face, pronator sign (pronation of hands when they are raised above the head), inability to keep the tongue protruded (“Jack in the box” tongue), and “milkmaid grip” when a child is asked to grip the examiner’s hand.
Epistaxis (acute rheumatic fever, pulmonic stenosis) and hemoptysis (tight mitral stenosis, pulmonary infarction, pulmonary edema) may occur due to underlying cardiac conditions. Hoarseness of cry in an infant may occur due to compression of the left recurrent laryngeal nerve as a result of dilatation of the main pulmonary trunk and pulmonary hypertension due to underlying congenital heart disease.
Ask for history of medications and status of penicillin prophylaxis in a patient suspected to have rheumatic heart disease. A history of recurrent chest infections is suggestive of left-to-right shunt. Patients with rheumatic fever may give a history of recurrent streptococcal sore throats. Ask about family history of consanguinity, congenital heart disease, and hypertension among siblings and parents.
There is an increased risk of congenital and even rheumatic heart disease among relatives as compared to the general population.Maternal diabetes mellitus is associated with hypertrophic cardiomyopathy with asymmetric septal hypertrophy, transposition of great vessels, and hypoplastic left heart syndrome in the offspring.
Ask for a maternal history of rubella, mumps, and other viral infections and intake of drugs during the first trimester of pregnancy which is characterized by organogenesis. Rubella syndrome is associated with patent ductus arteriosus while mumps during pregnancy may be associated with endocardial fibroelastosis in the fetus. Maternal systemic lupus erythematosus (SLE) is associated with an increased risk of complete heart block in the offspring.
General Physical Examination
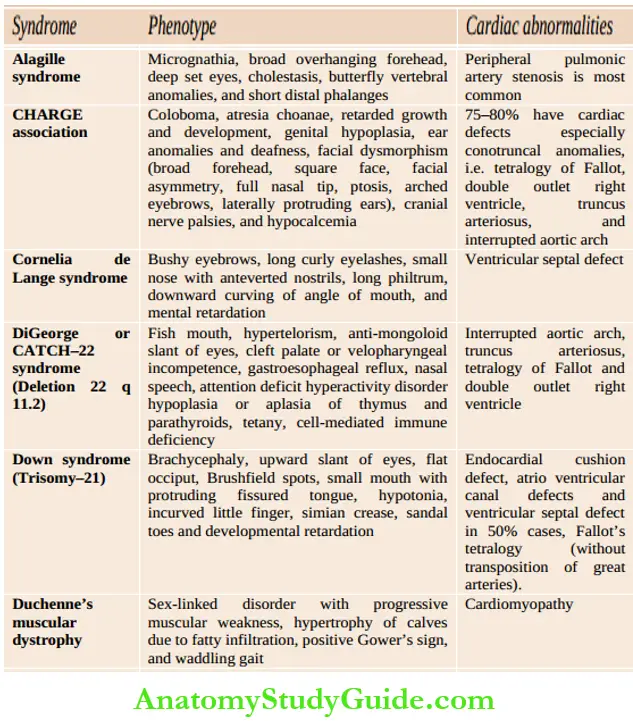
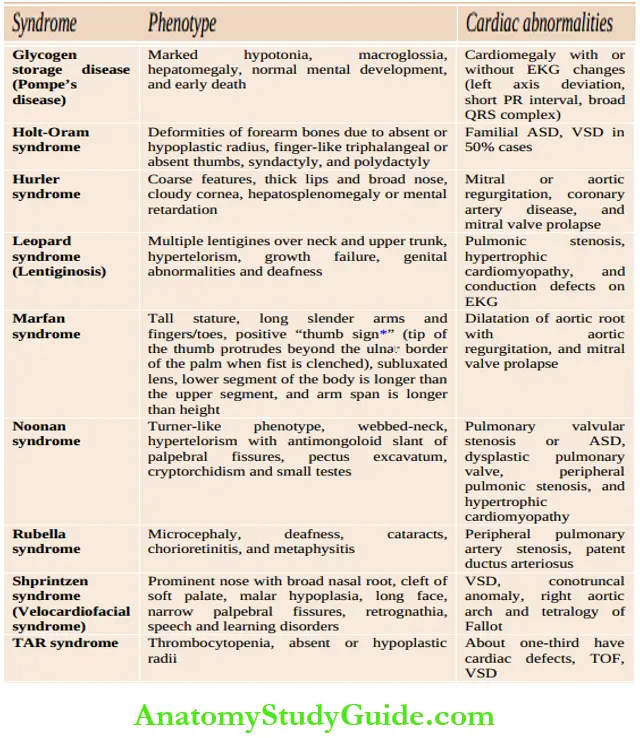
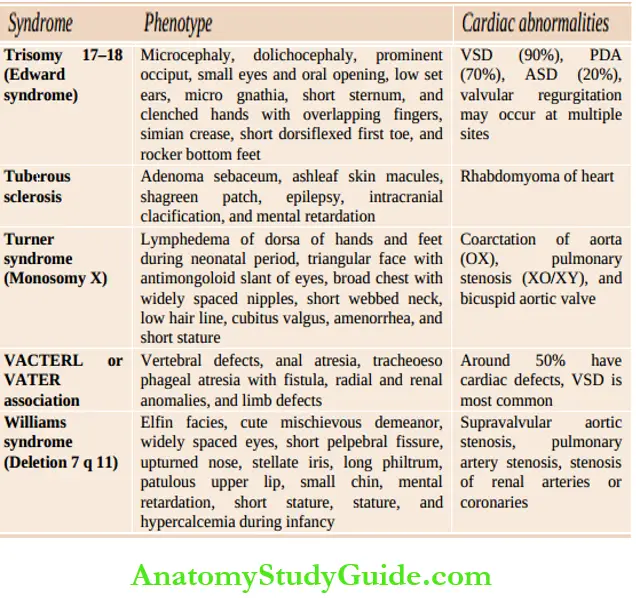
Assess whether the child is comfortable or dyspneic, evaluate the severity of dyspnea, posture (orthopnea), physical growth, and development. Look for peculiar phenotypes (Down syndrome, Turner syndrome, Marfan syndrome, Hurler syndrome, glycogenosis, etc.), malar flush, puffiness, and chorea outlines common cardiac malformations in association with chromosomal and developmental syndromes.
Pulse Radial pulse is examined with the tips of the index and middle fingers by gently compressing the vessel against the lower end of the radius. It is difficult to feel the radial pulse in newborn babies and infants. Pulse can be recorded from several other peripheral arteries, like brachial, carotid, femoral, popliteal, posterior tibial, and dorsalis pedis.
The carotid pulse should be felt in the groove infront of the sternocleidomastoid muscle while auscultating the heart. The presence of carotid thrill with an ejection systolic murmur in the right second intercostal space is diagnostic of aortic stenosis. The following characteristics of the pulse should be noted.
Rate (beats/min), volume (thrust), tension (force required to obliterate the pulse), rhythm (volume and rate), character (normal, bounding, collapsing or water hammer type, plateau), pulsus lemans (alternate stronger and weaker beat), pulsus bigeminy (alternate normal and ectopic beat), pulsus paradoxus, pulse on the other side and femoral (volume and temporal relationship with radial pulse) should be assessed .
Pulse pressure (systolic BPdiastolic BP) is wide (>40 mmHg) in children with aortic regurgitation, patent ductus arteriosus, arteriovenous fistula and thyrotoxicosis.
The pulse is low volume with narrow pulse pressure (<25 mm Hg) in patients with aortic stenosis, pericarditis, pericardial tamponade, and significant tachycardia. Assessment of vessel wall for its thickness is not important in children.
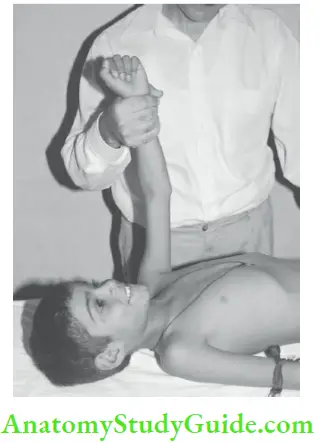
With the patient’s arm on the side, the wrist is so grasped that the radial pulse is barely palpable. When an arm is lifted up beyond the plane of the body, the radial pulse becomes readily palpable, if it is collapsing in character.
Common cardiac malformations in association with chromosomal and developmental syndromes:
Look for pulse deficit by simultaneously recording heart rate and pulse rate (ectopics and auricular fibrillation). The pulse may be regularly irregular due to an ectopic beat occurring at a regular interval or it may be irregularly irregular because of atrial fibrillation. Sinus arrhythmia in which the pulse rate becomes fast during inspiration and slows during expiration is common and physiological in children.
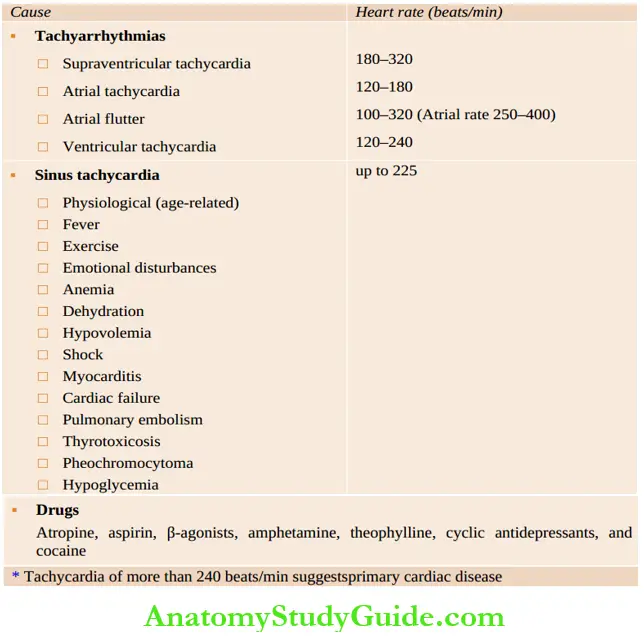
Pulsus alternans is characterized by alternating strong and weak beats due to severe dysfunction of the left ventricle. Tachycardia may occur from a large number of cardiac and systemic conditions . Bradycardia is diagnosed when the sinus rate is less than 90 beats/min in neonates and less than 60 beats/min in older children.
Pulsus paradoxus is a misnomer because it is an exaggeration of physiological findings wherein arterial pressure or pulse volume markedly diminishes or even disappears during deep inspiration. The systolic blood pressure is more than 10 mm Hg lower during inspiration as compared to expiration.
In a child who is unable to control his breathing on request, there is an alternative method to look for pulsus paradoxus. Ask the child to breathe normally and record his blood pressure. Note the reading on the manometer when the first Korotkoff sound is heard.
Allow the mercury to fall slowly until you hear the Korotkoff sounds loudly and continuously which gives a “doubling” effect to the sounds. Note the reading at this point and calculate the difference between the first and second readings. A difference of 10–20 mm Hg is equivocal but a difference of more than 20 mm Hg is highly suggestive of pulsus paradoxus. P
ulsus paradoxus is classically seen in patients with cardiac tamponade (pericardial effusion, constrictive pericarditis), restrictive cardiomyopathy, severe bronchial asthma, pneumothorax, and pleural effusion.
Causes of irregular pulse:
- Sinus arrhythmia
- Atrial extrasystoles
- Ventricular extrasystoles
- Atrial fibrillation
- Atrial flutter with variable response
- Second-degree heart block
Causes of Tachycardia in children:
Causes of bradycardia in children:
- Bradyarrhythmias: Sinoatrial block, AV block, complete bundle branch block, sick sinus
syndrome, and long QT syndrome - Athletic child
- Hypothermia
- Hypoxia
- Hypothyroidism
- Acidosis and electrolyte disturbances
- Raised intracranial tension
- Brainstem compression
- Excessive vagal tone
- Drugs and toxins: Digitalis, β-blockers, calcium channel blocker, intravenous calcium therapy, narcotics, clonidine, and organophosphates
- A sinus rate of less than 90 beats/min in neonates and less than 60 beats/min in older children is suggestive of sinus bradycardia
Temperature:
Fever may occur due to rheumatic activity, bacterial endocarditis, pulmonary infarction, and chest infection. Fever may also occur due to a cause unrelated to the cardiac condition.
Respiratory:
Rate Tachypnea is seen in CHF, anoxic spells, pulmonary emboli, and intercurrent respiratory infection.
Blood pressure:
Mercury or aneroid sphygmomanometers and automatic oscillometric devices are commonly used for recording blood pressure. A sphygmomanometer with a mercury column is more reliable than an aneroid instrument. It is conventionally recorded in the upper arm at the site of the brachial artery.
The child should be sitting with an arm kept at the level of heart or lying comfortably in bed. The appropriate size of the cuff which should cover two-thirds of the upper arm (or it should be 20% wider than the diameter of the upper arm or the width of the cuff should be 40% of the circumference of the arm) should be used.
The recommended cuff size in infants below one year of age is 2.5 cm, 1 – 4 years is 5 cm, 5 – 9 years is 9 cm and over 10 years is 13 cm. The use of a narrow blood pressure cuff is associated with spuriously high systolic blood pressure readings and vice versa.
The cuff is applied snugly over the upper arm by keeping its lower edge at least 2.0 cm above the cubital fossa. The manometer is kept at the same level as the cuff on the arm (heart level) and should be conveniently placed for the observer to view it.
Inflate the pressure in the cuff while palpating the brachial pulse. The level at which the brachial pulse disappears is a rough estimate of systolic blood pressure. Inflate the cuff further by another 20 – 30 mm Hg and listen through the diaphragm of the stethoscope placed lightly over the brachial artery. Deflate the cuff slowly by decrements of 2–3 mm Hg and the point when Korotkoff sounds are first heard is indicative of systolic blood pressure.
When cuff is further deflated, the Korotkoff sounds become muffled and finally disappear which is taken as an indication of diastolic blood pressure. The blood pressure reading is taken to the nearest 2 mm Hg. At times the Korotkoff sound does not disappear and in that case, muffling of the sounds is taken as the criterion for diastolic blood pressure A child is said to have hypertension if blood pressure is at or above the 95th centile for age and height.
The blood pressure should be recorded in both the upper limbs and the lower limbs when polyarteritis or coarctation of the aorta is suspected.
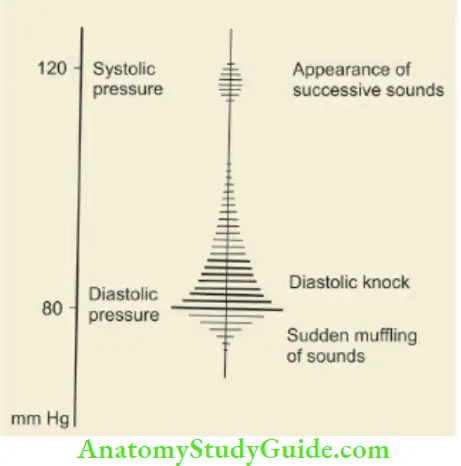
For recording lower limb blood pressure, the child is made to lie in the prone position (face down), the cuff is applied over the thigh and a stethoscope is placed over the popliteal artery in the popliteal fossa.
Normally, the lower limb systolic blood pressure is more by 10–30 mm Hg while the diastolic blood pressure is identical in the upper and lower limbs. In the coarctation of the aorta, the lower limb blood pressure is lower than the upper limb while in aortic regurgitation, the lower limb blood pressure is at least 40 mm Hg higher than the upper limb (Hill sign).
Pulse pressure is the difference between systolic and diastolic blood pressure and is indicative of pulse volume. In aortic regurgitation, the pulse pressure is high (≥80 mm Hg) while in aortic stenosis, the pulse is feeble with low pulse pressure.
Some adolescent children may complain of dizziness on standing due to postural hypotension. Check blood pressure in a relaxed supine position and record again after 2 minutes of standing. When there is ≥ 20 mm Hg drop in blood pressure on standing, it is suggestive of postural hypotension.
Criteria For Hypertension:
Age – 95th centile blood pressure (mm Hg)
Newborn – Systolic 95
7 days – Systolic 105
Upto 2 years – 112/74
3 – 5 years – 116/76
6 – 9 years – 122/78
10 – 12 years – 126/82
In infants and newborn babies, it is difficult to record blood pressure by the conventional method. Blood pressure can be recorded by the flush method. Cuff is wrapped around the upper arm, the limb is raised vertically and held above the head till the palm becomes pale. The pressure in the cuff is raised beyond the expected systolic blood pressure while maintaining the arm in a vertical position.
The arm is then brought down to the side on the cot and the cuff is gradually deflated. The point at which the palm becomes flushed is indicative of the systolic blood pressure of the infant. The diastolic blood pressure cannot be recorded by this method. In newborn babies and young infants, it is more convenient and reliable to use a non-invasive Doppler system to record blood pressure.
Anemia, cyanosis, jaundice (chronic liver congestion, pulmonary infarction, unrelated), lymphadenopathy, and edema should be looked for. Differential cyanosis with pink hands and blue feet may occur due to right-to-left shunt through patent ductus arteriosus because of severe pulmonary arterial hypertension.
Early and intense cyanosis (first week) should alert to the possibility of “T” diseases, like TGA, TAPVC, Tricuspid atresia, Truncus arteriosus, Total (critical) PS, and Tricuspid regurgitation.
Pink fingers with blue toes suggest that there is a normal connection of great vessels. When there is a complete transposition of great vessels, with pulmonary hypertension and reversed flow through the ductus arteriosus, the fingers will be more cyanosed than the toes.
Measurement of SaO2 in the right hand (pre-ductal) should be compared with the foot (post-ductal) to look for differential cyanosis. In newborn babies and infants with cardiac failure, sacral edema and puffiness may be seen while pedal edema is rare.
- Nails:
- Marked or drumstick clubbing is seen in children with cyanotic heart disease.
- Clubbing, splinter hemorrhages, (reddish-brown linear marks along the axis of nails),
- Osier nodes (tender red nodules over the finger pulps due to deposition of immune complexes), and
- Palmar ecchymosis (Janeway’s lesions) are indicative of bacterial endocarditis.
- Skin and joints:
- Look for subcutaneous nodules, erythema marginatum, chorea, and swelling of joints suggestive of rheumatic activity.
- However, fortunately, chorea and arthritis never coexist.
- Infants with CHF may have cold sweat on the forehead due to sympathetic overactivity as a consequence of decreased cardiac output.
- Examine joints and throat for any inflammatory signs.
- Fundus:
- Roth spots (flame-shaped hemorrhages with “cotton-wool” pale center) may be seen in children with bacterial endocarditis.
- In aortic regurgitation and patent ductus arteriosus, dancing retinal vessels are characteristic.
- Neck:
- Internal jugular vein is in direct communication with the right atrium and is best suited for the assessment of venous pressure and pulse waveforms.
- Look for jugular venous pressure (normal or raised), venous and arterial pulsations, and thrill.
- JVP is difficult to evaluate in infants due to short neck. In infants with congestive heart failure, scalp veins may become prominent and engorged.
- Examine thyroid gland for enlargement and bruit to rule out thyrotoxicosis.
- Venous pulsations:
- Venous pulsations in relation to various phases of the cardiac cycle are depicted in.
- The mechanism for the development of various individual waves in the internal jugular veins is shown in .
- As opposed to arterial pulsations, venous pulsations are not palpable and are less readily visible in an erect posture.
- Inspiration entrances venous pulsations by increasing venous return to the thorax.
- They are absent in children with auricular fibrillation, superior mediastinal obstruction, and constrictive pericarditis (prominent Y descent).
Giant ‘a’ waves or cannon waves are seen in tricuspid stenosis or atresia, pulmonary stenosis, pulmonary arterial hypertension, Ebstein’s disease, nodal rhythm, ectopic beats, and complete heart block. Giant V waves are seen in children with tricuspid regurgitation. a, c, and v and two negative waves or descents; x and y.
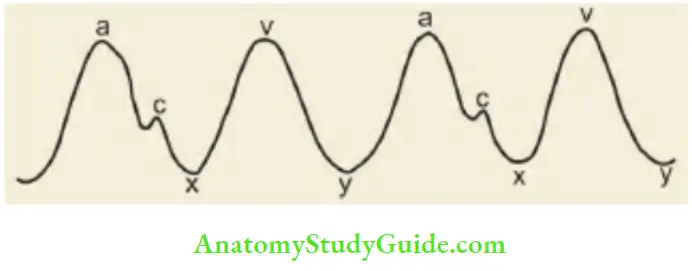
- The mechanisms for the development of individual waves in the internal jugular veins:
-
- a wave: Due to right atrial contraction
- c wave: Coincides with the onset of ventricular systole and results due to the movement of the tricuspid valve into the right atrium as the right ventricular pressure rises
- x descent: Due to atrial relaxation.
- v wave: Due to passive rise in the pressure as a venous return to the right atrium continues during ventricular systole while the tricuspid valve is closed
- y descent: There is lowering of right atrial pressure as the tricuspid valve opens and the blood rushes into the right ventricle
- Liver: Examine for liver size, tenderness, pulsations, and hepatojugular reflux (compression of the hepatic area is followed by engorgement of neck veins). Hepatojugular reflux is a sign of right ventricular compromise. Hepatomegaly is the most reliable sign of CHF in infants.
- Spleen: Splenomegaly may occur due to bacterial endocarditis or intercurrent infections, such as malaria or septicemia (typhoid fever).
Signs of Cardiac Failure:
Dyspnea, tachycardia, raised JVP, enlarged tender liver, edema, cardiomegaly, and basal crackles are recognized clinical signs of congestive heart failure (CHF). Jugular venous pressure is assessed by visualizing the engorgement of internal jugular veins in the neck.
The patient lies supine with their neck elevated by 45°. The vertical distance from the angle of Louis (sternal angle) to the imaginary line drawn from tire upper end of jugular venous column gives JVP which is measured in centimeters The sternal angle is taken as the reference point because the center of the right atrium is located at a depth of 5 cm behind the sternal angle in any position of the body.
When JVP is raised 3 cm above the sternal angle or the internal jugular vein is engorged above the level of clavicle, it is abnormal and suggestive of CHF, pericardial effusion, constrictive pericarditis, and mediastinal mass. In infants, evaluation of JVP is unreliable due to short neck and dependent edema is uncommon.
Hepatomegaly is the most consistent and reliable sign of CHF in infants. The central venous pressure can be measured by introducing a needle in the antecubital vein and connecting it to a manometer filled with saline. The normal CVP ranges between 4 and 6 cm H2O water and it is raised in CHF and reduced in hypovolemic shock.
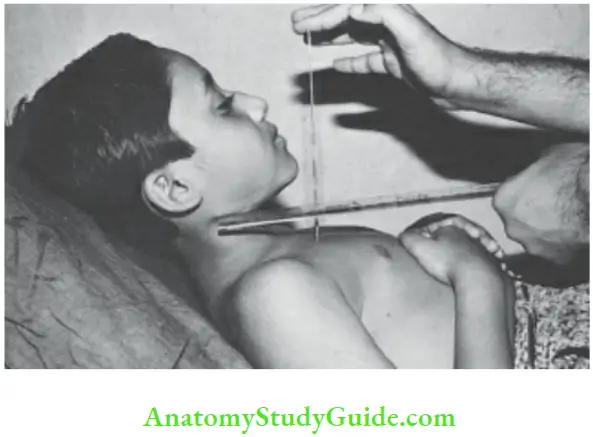
Child is made to lie in a Fowler’s position at an angle of 45°. The level of venous engorgement of the jugular vein in relation to the angle of Louis is measured with the help of two plastic rulers as shown in the photograph. When JVP is grossly elevated, neck veins may be engorged right up to the angle of the jaw even when a child sits up.
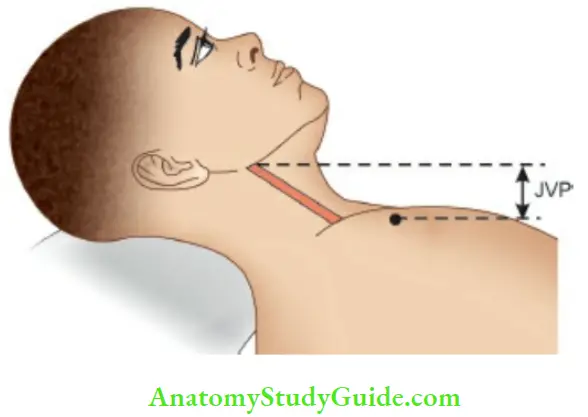
The child is placed in a reclining position at an angle of 45° so that angle of Louis corresponds to the base of the neck or upper margins of the clavicle. In this position, the height of the engorged right internal jugular vein is measured to assess JVP.
In infants, CHF indicates the presence of a severe underlying cardiac abnormality, like severe stenosis or atresia, left-to-right shunt, valvular regurgitation, and conditions with transposition physiology. CHF may be aggravated by underlying anemia, infective endocarditis, systemic hypertension, and coincidental myocardial disease.
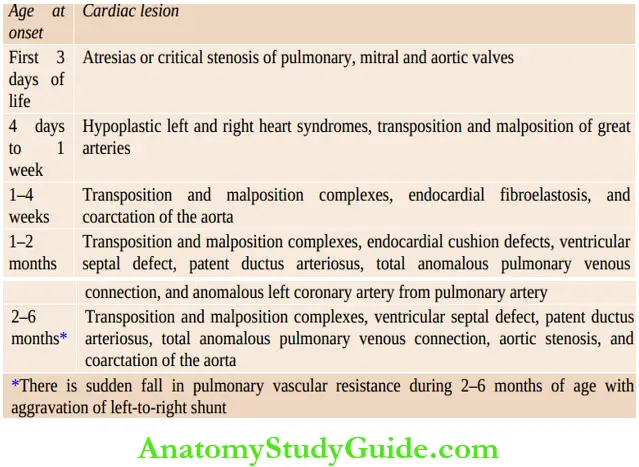
The time of onset of CHF is useful to suspect the nature of underlying congenital heart disease In most instances of congenital heart disease, CHF usually occurs dining the first 3 – 6 months of life. In fact, if there is no CHF dining the first year of life, it is unlikely to occur till the child is at least 10 years or older.
Time of onset of congestive heart failure in children with congenital heart disease:
Examination Of Heart
Inspection:
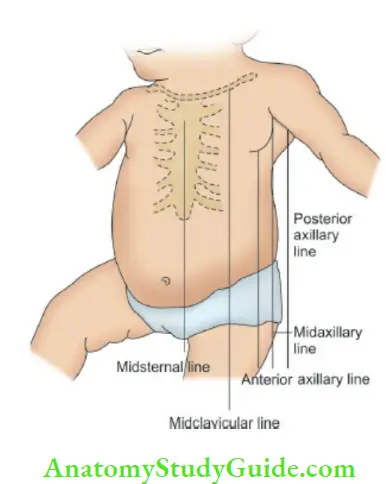
Bulging precordium (long-standing cardiomegaly), and bulging intercostal spaces (pericardial effusion) should be looked for. There are several thoracic lines of reference used in the examination of the heart to express the degree of cardiac enlargement
- Precordial pulsations:
- Note whether precordium is quiet (pericardial effusion, endocardial fibroelastosis, Ebstein disease), hyperdynamic or hyperkinetic (anemia, thyrotoxicosis, left-to right shunt, aortic and mitral regurgitation, etc.).
- Look for suprasternal and epigastric pulsations, dilated veins over the chest, and collateral arteries. Identify the site of the apex beat, if it is visible.
Palpation:
- Apex beat:
- It is the outermost and lowermost point of palpable impact of cardiac impulse.
- The apex beat is best palpated with the child sitting and leaning forward.
- The important landmark for counting the intercostal spaces is the angle of Louis or sternal angle.
- It is felt as a ridge connecting the manubrium sterni with the body of the sternum.
- The rib corresponding to the die sternal angle is the second rib and the space below is the die second intercostal space, hi preschool children’s apex beat is located in die 4th intercostal space just lateral to the die midclavicular line.
- In older children, it is located in the die 5th intercostal space inside or over the die midclavicular line.
- Assess whether it is visible or not (look on body sides), its location (up, down and out, outwards, inwards, or right side), and character (normal, feeble, tapping, heaving and hyperkinetic).
- Tapping apex is suggestive of palpable S in the mitral area while heaving (forceful, broad, and sustained) apex is indicative of left ventricular hypertrophy due to pressure overload.
The heaving apex with parasternal lift can be graded into 3 grades:
- Grade 1: Mild lifting of fingers and impulse can be obliterated,
- Grade 2: The lift is significant but not sustained and
- Grade 3: When lift is forceful and sustained.
Hyperkinetic apex beat is characterized by the exaggerated ill-sustained thrust of cardiac impulse and is seen in conditions associated with volume overload (anemia, aortic regurgitation, PDA, VSD, MR, and thyrotoxicosis). Left parasternal heave (due to right ventricular hypertrophy or conducted impulse from left atrial enlargement) and diastolic shock (palpable S2) should be looked for.
At times right parasternal heave may be felt if the right atrium is hugely dilated (Ebstein disease). Pulsations may be felt over the interscapular and infrascapular region due to dilatation of collateral vessels in children with coarctation of the aorta, pulmonary atresia, and Blalock-Taussig shunt.
The point of maximal impulse (PMI) is helpful in determining whether the right or left ventricle is dominant, hi patients with left ventricular dominance, the impulse is maximal at the apex whereas in right ventricular dominance the cardiac impulse is maximal over the lower left sternal border.
- Thrill: Vibratory sensations of heart musculature conducted through the chest wall (like the purring of a cat) when assessed on palpation is called thrill. It is associated with a murmur of Grade IV or higher. Note the size and timing of the thrill. The presence of a thrill is more certain evidence of underlying organic disease of the heart as opposed to the presence of a murmur alone. Functional murmurs are never associated with a thrill.
Percussion:
It has a limited clinical utility and is used to outline cardiac borders and to assess the heart size, high pericardial effusion, the dullness extends beyond the apex, and there is dullness over the 2nd left intercostal space which disappears when the patient sits up, i.e. shifting dullness (cf pulmonary hypertension).
In pulmonary hypertension and dilatation of pulmonary conus, the 2nd left intercostal space is dull both in supine and sitting positions. Dullness over the right second intercostal space and manubrium sterni may be seen in the aortic aneurysm and mediastinal mass (thymus).
Auscultation:
There is nothing criminal about the student’s use of the stethoscope as a status symbol but your status will suffer if your symbol lets you down. Admittedly, the most important part of the stethoscope is the part between the ears – your brain —John Apley.
After inspection, it is desirable to auscultate the heart in a sleeping infant before he is frightened or awakened by palpation and percussion. Offer a toy, key ring or your finger to the infant while auscultating to prevent him fiddling with the tubing of the stethoscope.
The bell-type chest piece is better suited for detecting low-frequency or low-pitched events (mitral diastolic murmur, third heart sound, and gallop rhythm) whereas the diaphragm selectively picks up the high-frequency sounds and murmurs.
Good quality stethoscope with short tubes (about 12 inches in length) and snugly fitting rubber earpieces, waxless ears, and intact drums of an experienced examiner, quiet infant, and serene surroundings are crucial correlates for optimal auscultatory yield.
Auscultatory findings are subjective and need experience and expertise on the part of the physician. Auscultation technology is likely to be revolutionized in the near future by the introduction of a stethoscope that is connected to a mobile smartphone or iPhone. The availability of echocardiography has deflated the ego of many cardiologists.
The defeatist attitude, however, should be replaced by the desire to improve and sharpen clinical acumen with the help of available imaging technology.
Auscultation should be done before palpation and percussion.
Mitral (apex), tricuspid (above xiphoid cartilage), 3rd and 4th left parasternal intercostal spaces, pulmonary (second intercostal space adjacent to the left sternal border) and A (second intercostal space adjacent to the right sternal border) area, neck vessels, sides and back of the chest and thyroid gland should be auscultated .
Auscultation should be done with the patient in the supine, upright, and left lateral positions and leaning forward. It is best to auscultate all areas of the heart for the quality and intensity of heart sounds and murmurs. The findings should be synthesized, reconciled, and expressed keeping in mind their character, intensity, “best heard” sites, and radiation
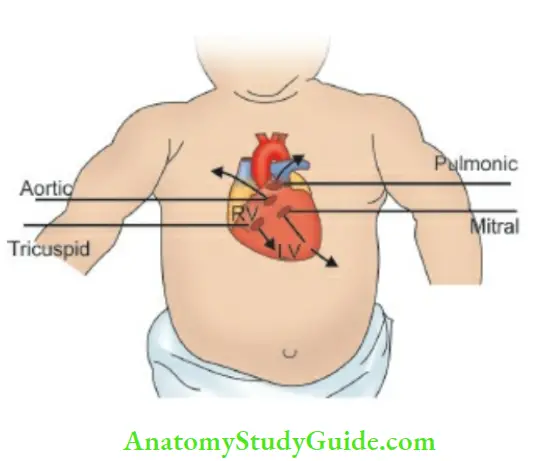
The heart sounds follow the direction of blood flow. The four standard cardiac areas project from the anatomical positions of cardiac valves as shown by arrows.
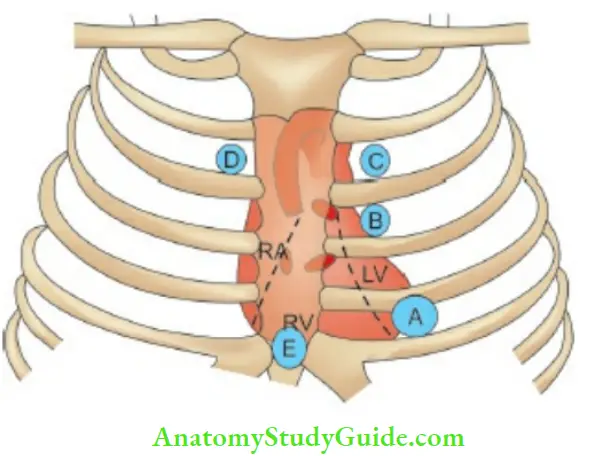
- (A) Mitral area
- (B) 3rd and 4th left intercostal space
- (C) Pulmonary area
- (D) Aortic area
- (E) Tricuspid area.
A protocol should be developed to describe auscultatory findings:
- The first and second heart sounds
- 3rd and 4th heart sounds in diastole
- Clicks and opening snaps
- Pericardial rub
- Murmurs in systole and/or diastole
Heart sounds:
The temporal relationship between events in the cardiac cycle and the production of heart sounds and jugular venous pulsations are diagrammatically depicted in The events on the two sides of the heart are asynchronous, the left ventricle starts contracting before the right ventricle.
The normal first heart sound is low-pitched, louder in intensity, longer in duration and the pause preceding it is longer than that following it. The first heart sound (closure of mitral and tricuspid valves) is best studied in the mitral area while the second heart sound (closure of aortic and pulmonary valves) is best heard in the pulmonary and aortic areas.
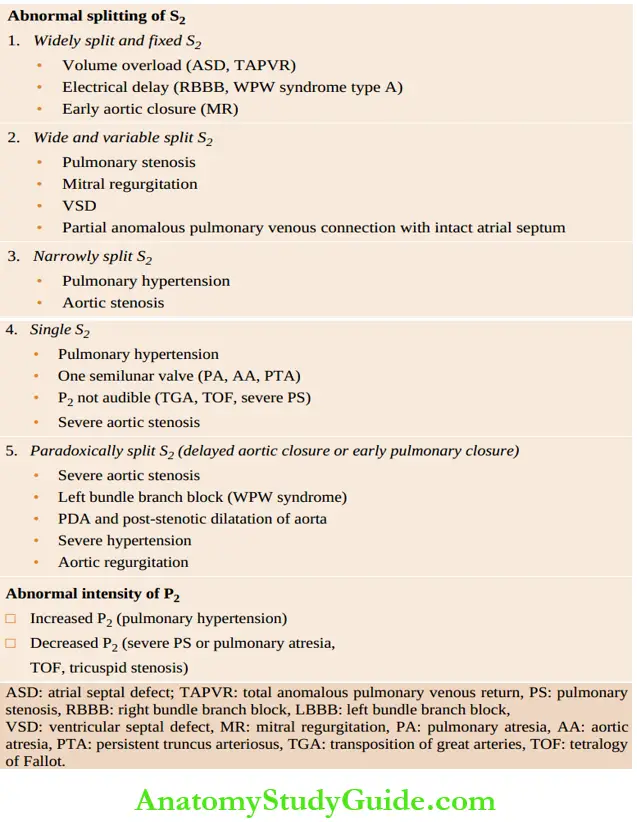
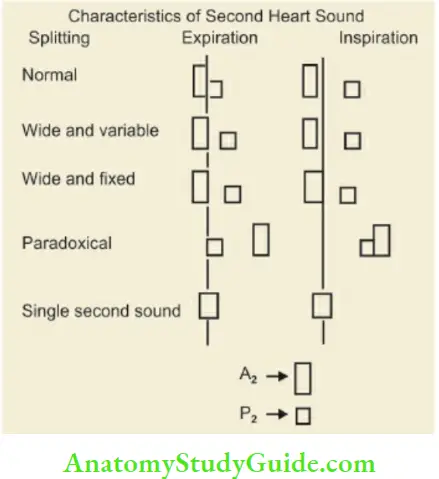
The second heart sound has two components, the aortic closure sound (A2) and the pulmonary closure sound (P2). During expiration, the two components are superimposed on each other resulting in a single-second heart sound. During the inspiratory phase of respiration, A2 occurs slightly early while P2 is delayed resulting in split S2, P2 is normally as loud as A2 and split (especially during inspiration) in children. P2 is louder than A2 up to 3 – 6 months of age.
Proper evaluation of S2 provides diagnostic clues in a number of clinical situations. When the split is heard both during inspiration and expiration, it is called a wide split. It is designated as a wide and variable split when the degree of splitting varies in inspiration and expiration; and a wide fixed split when it does not vary during inspiration and expiration, hi reversed or paradoxical split, P2 occurs earlier than A2.
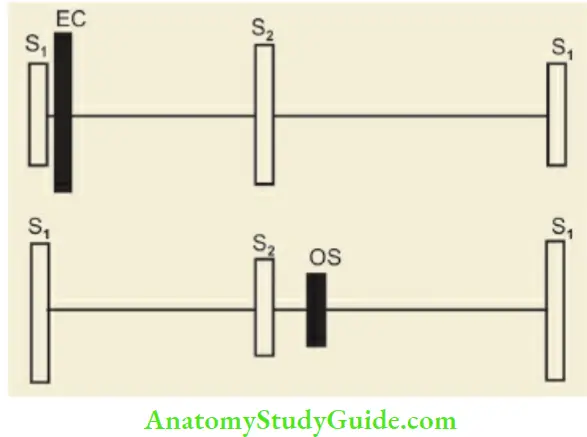
The opening of normal cardiac valves does not produce any sound but when they are diseased they may produce ejection clicks. The opening snap is a high-frequency sound heard in diastole immediately after the second heart sound and is indicative of a stenotic mitral valve with a pliable and mobile anterior leaflet. It is best heard in expiration at the fourth intercostal space over the left lower sternal border.
Look for intensity of heart sounds, whether relative and absolute, splitting (fixed or variable), 3rd heart sound (triple or gallop rhythm due to rapid filling of ventricles during early diastole), opening snap (snappy sound inside the apex that closely follows the second heart sound due to opening of the stiff mitral valve), and ejection or systolic click (opening of diseased pulmonary and aortic valves) .
Fourth heart sound which represents atrial contraction may be heard in patients with severe pulmonic stenosis, pulmonary hypertension, and Ebstein disease. It is a low-pitched sound occurring before S1 and is rarely heard in children. Third and fourth heart sounds are best heard when the patient is turned to the left side and auscultated with the bell of the stethoscope.
The heart sounds and murmurs should be diagrammatically charted as shown in. The significance and mechanism of production of abnormal and extra heart sound is shown in The cardiac cycle.
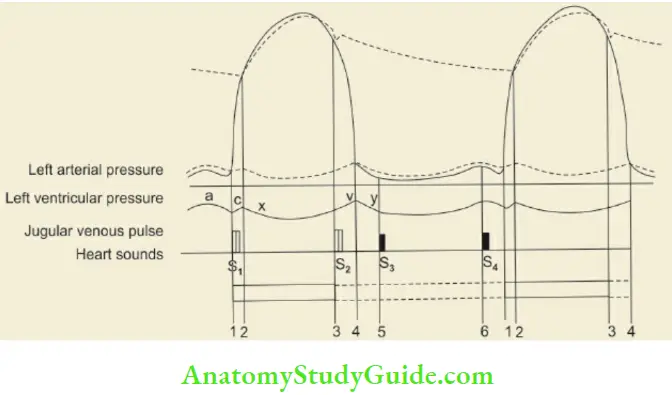
Note: The temporal relationship of jugular venous pulsations with heart sounds and cardiac systole and diastole
Clinical significance of abnormal S2:
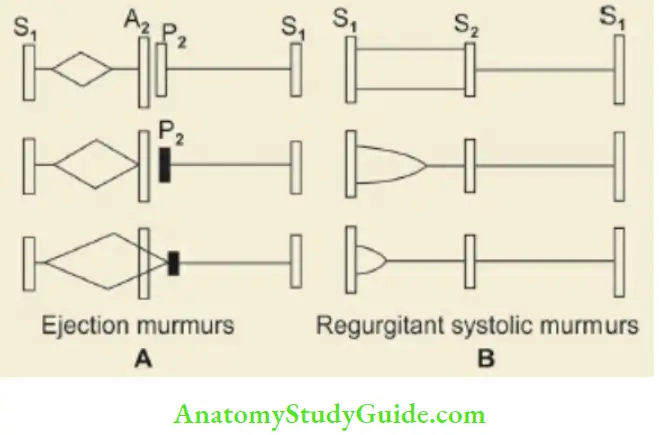
Cardiac murmurs are produced due to turbulence of the blood flow at or near a valve or because of abnormal communication within the heart. Site of maximum intensity, grade (1– 6/6), timing (systolic, diastolic or continuous), character and quality (ejection holosystolic or pan-systolic, decrescendo or crescendo and continuous type) and radiation or conduction should be carefully evaluated.
The murmurs are timed in relation to first and second heart sounds and by simultaneous palpation of apex beat or carotid artery. Some murmurs are best heard in certain postures and phases of breathing. Murmurs originating from the right side of the heart increase in intensity during inspiration owing to increase in stroke output of the right ventricle.
Conversly murmurs arising from the left side are accentuated during expiration. All murmurs increase in intensity with forceful maneuvers, like hand grip except mitral valve prolapse and idiopathic hypertrophic subaortic stenosis. Positions and maneuvers for the best elicitation of certain murmurs are given below.
Conduction or radiation of murmurs;
- Localized murmurs: Mitral stenosis, pulmonary and tricuspid valve lesions
- Mitral regurgitation: Left axilla and left the infrascapular area
- Aortic stenosis: Carotid arteries and apex
- Patent ductus arteriosus and pulmonary stenosis: Back
- Ventricular septal defect: Right sternal edge
Mitral stenosis: Left lateral position (bell of stethoscope)
Aortic regurgitation: Child sitting, leaning forward, and during expiration (diaphragm or chestpiece of stethoscope).
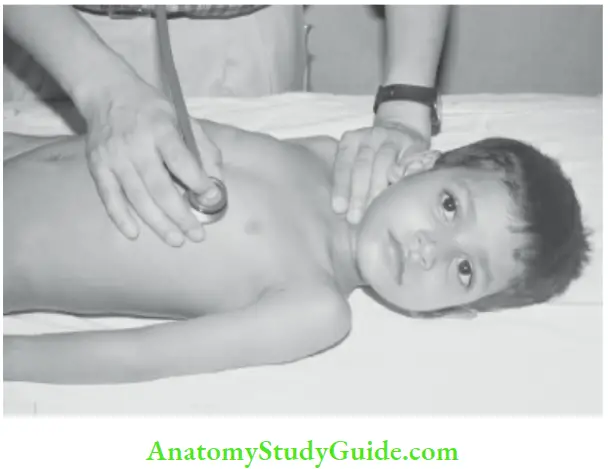
Significance and mechanism of production of abnormal heart sound:
Tricuspid regurgitation: End of deep inspiration.
The intensity of murmur is conventionally graded from I to VI.
- Grade I: Barely audible;
- Grade II: Soft but easily audible;
- Grade III: Loud but not accompanied by a thrill
- Grade IV: Louder and associated with thrill.
- Grade V: Audible with stethoscope barely touching the chest: Grade VI audible with stethoscope off the chest or naked ear.
Grading of murmur:
- Grade I – Soft, audible to an experienced cardiologist
- Grade II – Soft but clearly audible
- Grade III – Easily audible murmur without any thrill
- Grade IV – Loud murmur associated with a thrill
- Grade V – A very loud murmur, audible even when the diaphragm of the stethoscope is barely touching the chest
- Grade VI – Audible directly by naked ear or diaphragm lifted off the chest
The intensity of the murmur may not suggest the severity of underlying heart disease. For example, a small VSD may produce a loud flow murmur while a large aortic regurgitation may produce an inaudible flow murmur.
A neonate with complex congenital cardiac malformation may die without manifesting any significant cardiac murmur. No murmur is usually audible in patients with transposition of the great arteries, double outlet right ventricle, cardiomyopathy, and arrhythmias.
The presence of a continuous murmur in a cyanotic patient is a recognized feature of patent ductus arteriosus, rupture of sinus of Valsalva fistula into the right atrium or ventricle, systemic AV fistula, coarctation of the aorta, peripheral pulmonary stenosis, aortopulmonary septal defect, anomalous left coronary artery from the pulmonary artery, mitral stenosis with small atrial septal defect and venous hum.
The continuous murmur must be differentiated from pericardial friction rub. The characteristics of cardiac murmurs are summarized in In prosthetic valves, the opening and closing sounds are louder with a clicking or metallic quality. They may be associated with a mid-systolic murmur but the presence of a diastolic murmur in association with a prosthetic valve suggests a leak.
Venous hum:
It may be present in 40–50 percent of children below the age of 5 years. It is a faint continuous hum best heard at the first or second right interspaces adjacent to the sternum. The hum disappears when neck vessels are obliterated by applying finger pressure just above the medial end of right clavicle.
Characteristics of the murmur:
- Timing: Systolic, diastolic, or continuous
- Character: Blowing, rumbling, rough and rasping, ejection systolic or ‘diamond-shaped
- Intensity: Grade I-VI
- Conduction or selective propagation
- Change with breathing: Right heart murmurs increase with deep inspiration (tricuspid regurgitation) while left heart murmurs decrease with inspiration.
- Valsalva maneuver: The murmurs of mitral valve prolapse and hypertrophic obstructive cardiomyopathy increase in intensity
- Change with position: Most murmurs diminish in intensity on sitting or standing except murmurs of mitral valve prolapse, hypertrophic obstructive cardiomyopathy, aortic regurgitation, and soft systolic murmur of acute rheumatic fever
- Exercise: Most murmurs increase in intensity after exercise except murmurs due to hypertrophic obstructive cardiomyopathy and innocent murmurs
- Effect of systemic arterial resistance: When peripheral vascular resistance is increased by applying a blood pressure cuff on both upper arms and inflating it beyond systolic pressure, it increases the intensity of murmurs due to mitral regurgitation, aortic regurgitation, and ventricular septal defect
- Valsalva maneuver: Ask the child to blow or strain against closed glottis (as if trying to pass a constipated stool) so that intrathoracic pressure goes up Pericardial rub It is a leathery to-and-fro, superficial creaky sound, variable in intensity, and heard in both phases of cardiac contraction and altered by the pressure of chest piece. It is best heard at the base of the heart or inside the apex especially when the patient leans forwards.
Diagnostic Clinical Signs Of Common Cardiac Conditions
Mitral Stenosis:
Feeble pulse, left parasternal heave, tapping apex, palpable S2, diastolic thrill, lound S1 in mitral area, opening snap (occurring shortly after the second heart sound), middiastolic rough and rumbling murmur with presystolic accentuation are characteristic clinical findings.
The absence of late diastolic accentuation of murmur goes against the diagnosis of significant mitral stenosis. The presystolic component disappears during auricular fibrillation. A loud first heart sound and opening snap indicates the presence of a relatively pliable non-calcified valve.
The closeness of the opening snap to the second heart sound and the duration of diastolic murmur are useful indicators of the severity of mitral stenosis. A short mid-diastolic rumble (Carey-Coomb murmur) due to mitral valvulitis may be heard in patients with acute rheumatic carditis without any established mitral stenosis.
Mitral Regurgitation:
Pulse is a good volume with wide pulse pressure. Heaving and hyperkinetic apex, systolic thrill in mitral area, muffled or inaudible S1, with high-pitched pansystolic murmur (murmur starts immediately after the first heart sound and continues up to and through the second heart sound) which is conducted towards the axilla and back are suggestive of mitral regurgitation, hi most cases, a third heart sound is audible at the apex and indicates early rapid filling of the left ventricle.
Mitral Valve Prolapse:
It is usually asymptomatic in children and characterized by an apical mid-systolic click, a high frequency usually loud intensity sound with a scratchy quality which is best heard in a sitting or standing position. There may be a mid-late Grade II systolic murmur with a whistling character.
The abnormality is commonly seen in young girls who may complain of palpitations. The prevalence of mitral valve prolapse is increased in patients with Duchenne muscular dystrophy, osteogenesis imperfecta, Ehlers-Danlos syndrome, Marfan syndrome, von Willebrand disease, fragile X syndrome, and anorexia nervosa.
Tricuspid Regurgitation:
Prominent venous pulsations (giant ‘v’ waves followed by a large ‘y’ descent) in neck, pulsatile liver, high-pitched pan systolic murmur at lower end of the sternum accentuated by deep inspiration are characteristic. Functional tricuspid regurgitation due to right ventricular failure is associated with a third heart sound. It is usually associated with signs of pulmonary hypertension and mitral valve disease.
Aortic Regurgitation:
There are features of “aortic rim off” like collapsing or water hammer pulse (Corrigan pulse), wide pulse pressure, prominent arterial pulsations, prominent carotid pulsations (Corrigan’s sign), pistol shot sounds, forceful heaving apex, decrescendo high-pitched, blowing and early diastolic murmur in the aortic area.
In severe aortic regurgitation, nodding of the head may occur with each systole (deMusset’s sign) due to sudden filling of carotid vessels. The diastolic murmur is best heard with the diaphragm of the stethoscope over the left upper parasternal area when the patient sits up, leans forwards and breathes out.
The increased flow of blood across the aortic valve may produce an ejection systolic murmur. The first heart sound is soft and the aortic component of the second sound is delayed and accentuated. In severe aortic regurgitation, a lowpitched diastolic flow murmur called as Austin-Flint murmur may be audible at the mitral area.
This murmur originates from the anterior leaflet of the mitral valve due to the confluence of two streams of blood, i.e. antegrade flow from the left atrium and regurgitation stream from incompetent aortic valve.
There is an exaggeration of the systolic pressure difference between brachial and femoral arteries (Hill’s sign). When systolic pressure in the femoral artery is 40–60 mm Hghigher than upper limb pressure, it is indicative of moderate-severe aortic regurgitation.
Aortic Stenosis:
Plateau or feeble pulse, narrow pulse pressure, heaving apex, systolic thrill over the aortic area conducted to neck vessels, a delayed aortic component of S2, a “diamond-shaped” ejection systolic murmur conducted to neck vessels are characteristic findings of aortic stenosis.
Ejection clicks may be audible just after the first heart sound in valvular aortic stenosis. The S2 may be single or paradoxically split and pulse pressure is extremely narrow. S2 and S4 may be heard in severe aortic stenosis. Williams syndrome is characterized by peculiar “elfin facies”, hypercalcemia, mental retardation and supra valvular aortic stenosis.
Atrial Septal Defect (ASD Secundum):
Parasternal heave, grade 2 – 3 ejection systolic murmur over 2nd and 3rd intercostal spaces, and fixed wide splitting of S2, are diagnostic features of ASD secundum. The murmur is produced by the increased flow of blood through the pulmonary valve and not due to the left-to-right shunt.
The murmur may be widely transmitted all over the chest and may be associated with a delayed diastolic murmur at the lower left sternal border. Cardiomegaly is mild and cardiac failure is rare. Marked cardiomegaly suggests the existence of additional lesions, like mitral valve obstruction (Lutembacher syndrome) or mitral regurgitation (ostium primum defect or associated rheumatic mitral regurgitation).
Endocardial Cushion Defect (Ostium Primum Defect):
The defect in the atrial septum is situated below the fossa ovalis and is associated with a cleft in the anterior leaflet of the mitral valve. In addition to the findings of ostium secundum listed above, there are additional clinical features suggestive of left ventricular hypertrophy, pan systolic murmur of mitral regurgitation, and left axis deviation of more than – 30° on EKG.
Congestive heart failure is common in children with the common atrioventricular canal. Endocardial cushion defect is common in children with Down syndrome.
Ventricular Septal Defect:
Wide pulse pressure, hyperdynamic precordium with forceful apex beat, systolic thrill over the left sternal border, pan systolic murmur over the left sternal region (3rd to 5th interspace), masking of both heart sounds are usual clinical findings. S2 may be split with the accentuation of P2. A third heart sound may be audible at the apex.
A diastolic flow murmur in the mitral area may be heard in children with a large defect. Children with VSD become symptomatic around 6 – 10 weeks of age when pulmonary pressure becomes lowest.
Pulmonic Stenosis Prominent ‘a’ waves in jugulars, parasternal heave, wide and variably split S2, harsh grade 2 – 5/6 ejection systolic murmur, and systolic ejection click best heard during expiration, are recognized clinical features of pulmonary stenosis. The intensity and duration of tire murmur and delay in tire pulmonary component of S2 are suggestive of the severity of pulmonary stenosis.
Patent Ductus Arteriosus (PDA):
Collapsing pulse, heaving apex, systolic thrill below tire left clavicle, machinery or rolling thunder harsh continuous systolic-diastolic murmur best heard over 2nd left intercostal space, and multiple clicks are recognized clinical findings of PDA.
The first sound is accentuated and S2 is narrowly or paradoxically split with large left-to-right shunt. Pulmonary arteriovenous fistulae, coronary arteriovenous anastomoses, aorticopulmonary fenestration, and venous hum may produce continuous murmurs. A diastolic murmur in the mitral area may appear due to large blood flow across the mitral valve.
Tetralogy of Fallot:
Fallot’s tetralogy consists of pulmonary stenosis, ventricular septal defect, right ventricular hypertrophy, and over-riding of the aorta. Cyanosis, clubbing, anoxic spells, prominent ‘a’ waves in the jugular venous pulse, mild right ventricular hypertrophy, ejection systolic murmur over the pulmonary area, single-second heart sound (absent pulmonary component), and absence of congestive heart failure are suggestive of tetralogy of Fallot. Anoxic spells and squatting are common in children with TOF.
Tricuspid Atresia:
Cyanosis, anoxic spells, prominent ‘a’ waves in jugular veins, presystolic pulsations in the liver, the apical impulse of left ventricular type, and non-significant or absent cardiac murmur are usual clinical findings. It may be difficult to clinically differentiate these patients from tetralogy of Fallot.
Transposition of Great Vessels:
Cyanosis with CHF since early infancy, right ventricular hypertrophy, loud and single S2 with a systolic ejection click, short systolic ejection murmur of pulmonary stenosis or systolic regurgitant murmur of VSD, or no murmur are recognized clinical features of transposition of great vessels.
Ebstein Anomaly:
Cyanosis, clubbing, dominant V waves in the neck, quiet precordium, left ventricular type apical impulse, normal or split S1, tricuspid opening snap, triple or quadruple heart sounds (multiple sounds are audible), systolic thrill with mid or pan systolic murmur at the left sternal border are suggestive of Ebstein’s anomaly. There may be delayed short diastolic murmur at the tricuspid area. Both systolic and diastolic murmurs produced at the tricuspid valve may have a scratchy character not unlike a pericardial friction rub.
Coarctation of Aorta:
The blood pressure proximal to the stricture, i.e. upper limbs is increased so that blood pressure in the arms is higher than the lower limbs. Femorals are feeble and delayed as compared to simultaneously felt radial or brachial pulsations. Upper arms are stronger and span may be longer than the height.
Palms may appear pinker as compared to soles. Collateral vessels linking the subclavian arteries and intercostal arteries may be seen or felt over the interscapular and infrascapular areas. Collaterals are palpable over the undersurface of ribs on the back and along both the borders of the scapulae.
Precordial examination shows evidence of left ventricular hypertrophy, aortic ejection systolic murmur (due to associated congenital bicuspid aortic valve) and constant ejection click.
Ejection systolic or continuous murmur may be audible over the back due to the presence of collaterals or the flow of blood through the narrow segment of the aorta (Suzman sign).
Myocarditis:
There is cardiomegaly, marked tachycardia, and low-intensity heart sounds with gallop rhythm. At times, a “tic-tac” rhythm in which the interval between the first and second heart sounds is equal to or even longer than diastole may be heard. The regurgitant murmur may be produced by gross enlargement of the heart with dilatation of the valves.
Dysrhythmias in the form of ventricular premature beats or conduction disturbances are common. Evidence of congestive heart failure are usually present.
Functional Cardiac and Extracardiac Murmurs:
Transient, nonsignificant murmurs are common during the newborn period due to post-natal delay in circulatory adaptation. The closure of the ductus and foramen ovale may be delayed. Conversely, infants with VSD may not have any murmur during neonatal period which may appear after 4 to 6 weeks of age when pulmonary vascular resistance falls leading to the establishment of left-to-right shunt.
The “functional” or “innocent murmurs” are best heard at the base, are low-pitched and low in intensity, systolic in timing, unassociated with thrill, modified by the posture of the patient and usually disappear following exercise All innocent murmurs are accentuated by high-output states, like fever and anemia, and are associated with normal EKG and skiagram of the chest.
Anemic children with a cardiac murmur should be reexamined after correction of anemia before any pathologic significance is ascribed to the murmur. Functional murmurs may also occur in children with fever, scoliosis, kyphoscoliosis, and pectus excavatum.
Characteristics of innocent murmurs:
- Predisposing factors: Early age, fever, anemia, chest or spinal deformity
- Ejection systolic low-pitched murmur Grade II or less, best heard in an upright position. No thrill is palpable.
- Murmur is modified and may disappear after exercise, change of position or rotation of the head, occlusion of neck veins or by increasing pressure over the chest piece of the diaphragm
- No diastolic murmur
- Normal EKG and X-ray chest
Venous hum is best audible at the right infraclavicular and supraclavicular areas as a continuous murmur (louder diastolic component) due to turbulence of blood in the jugular system especially in children between 3 and 6 years of age. The murmur is best heard in an upright position and disappears when a child lies supine or by rotation of the head.
The murmur also disappears, if neck veins are occluded with a finger or by increasing pressure over the chestpiece of stethoscope. A carotid bruit may be heard as an early ejection systolic murmur over the carotid arteries. Unlike aortic stenosis, there is no ejection click and no thrill or murmur is heard over the aortic area.
In children with coarctation of aorta, look for murmur over the back due to collaterals. Auscultation of the skull, liver, and lumbar regions is advocated in children with unexplained CHF and hypertension. A continuous or ejection systolic murmur may be audible due to arteriovenous fistula, angiomatous malformation and arterial stenosis.
The murmur is considered as pathological if the child has cyanosis and there are features suggestive of cardiac failure. The presence of heart disease can be suspected on the basis of Nadas criteria The presence of one major or two minor criteria is suggestive of underlying heart disease.
Nadas’ criteria for the presence of heart disease:
Major – Minor
Systolic murmur Grade III or more – Systolic murmur less than Grade III
Diastolic murmur – Abnormal second heart sound
Cyanosis – Abnormal EKG
Cardiac failure – Abnormal X-ray chest , Abnormal blood pressure
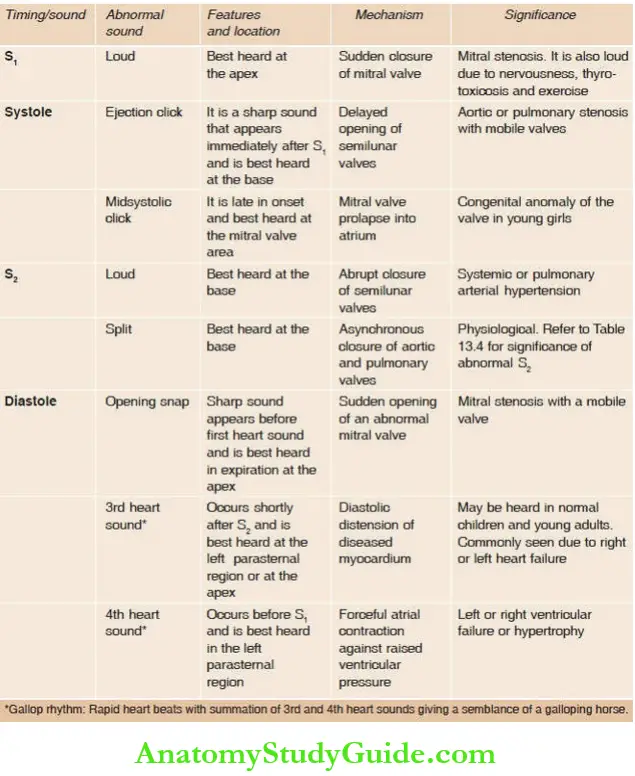
Scheme for Presentation:
Leave a Reply