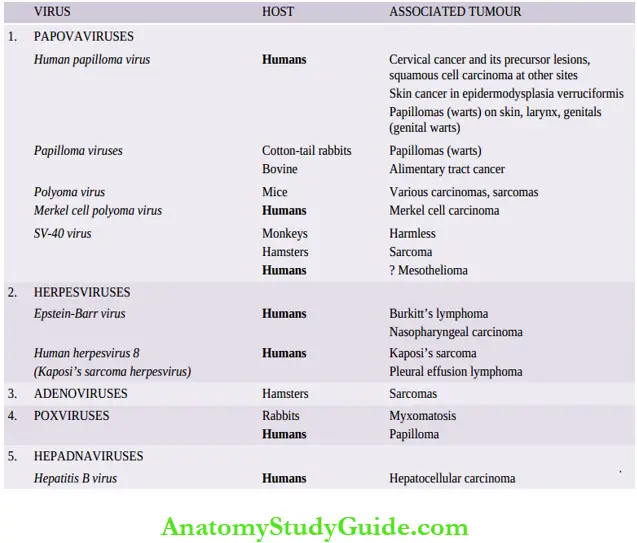
DNA Oncogenic Viruses
DNA oncogenic viruses have direct access to the host cell nucleus and are incorporated into the genome of the host cell. DNA viruses are classified into 5 subgroups, each of which is capable of producing tumors in different hosts.
Table of Contents
Read And Learn More Neoplasia
These are:
- Papovaviruses
- Herpesviruses
- Adenoviruses
- Poxviruses and
- Hepadna viruses.
Papovaviruses
This group consists of the papillomavirus including the human papillomavirus (HPV), polyomavirus and SV-40 (simian vacuolating) virus.
These viruses have an etiologic role in following benign and malignant tumours in animals and in humans:
- Papillomaviruses: Papillomaviruses were the first to be implicated in the etiology of any human tumour. These viruses replicate in the layers of stratified squamous epithelium. More than 100 HPV types have been identified; the individual types are associated with different lesions.
10 DNA oncogenic viruses:
- Papillomaviruses are implicated in the etiology of the following benign and malignant tumours:
- HPV was first detected as an etiologic agent in common skin warts or verruca vulgaris by Shope in 1933; the condition is infectious. Low-risk HPV types 1, 2, 4 and 7 are involved in these common lesions.
- Low-risk HPV types 6 and 11 are involved in the etiology of genital warts (condyloma acuminata).
- Viral DNA of high-risk HPV types 16, 18, 31, 33, and 45 is found in 75-100% cases of invasive cervical cancer and its precursor lesions (high-grade squamous intraepithelial lesions i.e.
- Carcinoma in situ and dysplasia) and is strongly implicated.
- High-risk HPVs are also involved in causing squamous cell carcinomas and dysplasias of other sites such as of anus, perianal region, vagina, vulva, penis, and oral cavity.
- HPV types 5 and 8 are responsible for causing an uncommon autosomal recessive condition, epidermodysplasia verruciformis. The condition is characterised by multiple skin warts and a genetic defect in the cell-mediated immunity.
- About one-third of cases develop squamous cell carcinoma in the sun-exposed warts.
- Some strains of HPV are responsible for causing multiple juvenile papillomas of the larynx.
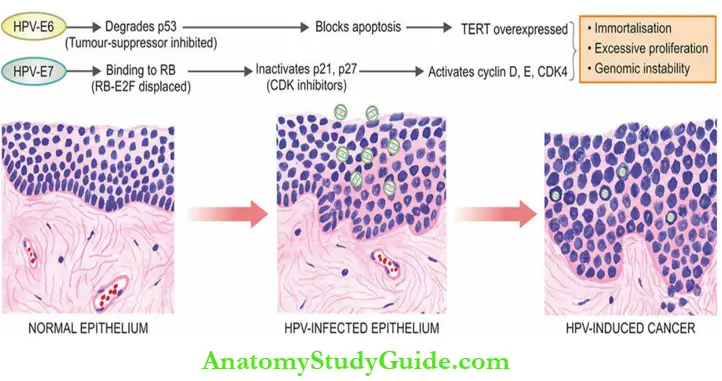
- Hpv Oncogenesis: On persistence of infection with high-risk HPV types, the viral DNA is integrated into the target epithelial cells.
- This results in genomic instability of the host cell leading to loss of E2 viral repressor and overexpression of viral oncoproteins E6 and E7.
- These oncoproteins (E6 and E7) from high-risk HPVs have high affinity for target host cells than these oncoproteins from low-risk HPVs.
- Transforming effects of HPV are largely due to alterations in genes encoding for E6 and E7 oncoproteins as under :
- Oncogenic effects of E6 These are:
- It degrades p53, and thus inhibits its tumor-suppressor effect.
- Degradation of p53 causes a block in apoptosis because p53 normally activates BAX, a proapoptotic gene.
- It stimulates the expression of the TERT (telomerase reverse transcriptase) catalytic subunit and thereby contributes to immortalisation of the cell.
- Oncogenic effects of E7 These are:
- It binds to RB protein and displaces its E2F transcription factor, thus removing the brake in
the cell cycle. - It inactivates CDK inhibitors p21 and p27, thus permitting further cell proliferation.
- It binds and activates cyclin D, E, and CDK4, promoting cell proliferation.
- In addition to the transforming effects of E6 and E7 oncoproteins derived from high-risk HPVs, a few cofactors play role in development of cervical cancer.
- These include Immunosuppression, cigarette smoking, other coexisting infections, hormonal changes, dietary deficiencies etc.
- It binds to RB protein and displaces its E2F transcription factor, thus removing the brake in
- Oncogenic effects of E6 These are:
- Polyomavirus: In view of its involvement in causing multiple unrelated tumors in animals, it was named polyoma.
- Merkel cell polyomavirus has been recently found involved in Merkel cell carcinoma, an infrequent cancer of the skin.
- It is also implicated in polyomavirus nephropathy in renal allograft recipients and progressive demyelinating leucoencephalopathy, a fatal demyelinating disease.
- SV-40 virus:
- Simian vacuolating (SV) virus exists in monkeys without causing any harm but was found in cell cultures being prepared for human polio vaccine in 1960.
- It was subsequently found that SV-40 could induce sarcoma in hamsters but not in humans.
- There is some evidence of involvement of SV-40 infection in mesothelioma of the pleura.
Herpesviruses
Primary infection of all the herpesviruses in man persists probably for life in a latent stage which can get reactivated later. Important members of herpesvirus family are
Epstein-Barr virus, herpes simplex virus type 2 (HSV-2) and human herpesvirus 8 (HHV8), cytomegalovirus (CMV).
There is no oncogenic role of HSV-2 and CMV in human tumors. The other two—EBV and HHV are implicated in human tumors as under.
Epstein-Barr Virus (EBV):
EBV infects human B-lymphocytes and epithelial cells and long-term infection stimulates them to proliferate and develop into malignancies of corresponding cells. EBV is implicated in the following human tumors:
- Burkitt’s lymphoma
- Anaplastic nasopharyngeal carcinoma
- Post-transplant lymphoproliferative disease
- Primary CNS lymphoma in AIDS patients, and
- Hodgkin’s lymphoma.
EBV is also etiologic agent for infectious mononucleosis, self-limiting disease in humans Oncogenic role of two of common EBV-induced tumours (Burkitt’s lymphoma and anaplastic nasopharyngeal carcinoma) is given below.
Burkitt’s lymphoma:
Burkitt’s lymphoma was initially noticed in African children by Burkitt in 1958 but is now known to occur in 2 forms—African endemic form, and sporadic form seen elsewhere in the world. The morphological aspects of the tumor.
There is strong evidence linking Burkitt’s lymphoma, a B-lymphocyte neoplasm, with EBV as observed from the following features:
- Over 90% of Burkitt’s lymphomas are EBV-positive in which the tumor cells carry the viral DNA.
- 100% cases of Burkitt’s lymphoma show elevated levels of antibody titres to various EBV antigens.
- EBV has strong tropism for B lymphocytes. EBV-infected B cells grown in cultures are immortalized i.e. they continue to develop further along B-cell line to propagate their progeny in the altered form.
- Though EBV infection is almost worldwide in all adults and is also known to cause self-limiting infectious mononucleosis, the fraction of EBV-infected circulating B-cells in such individuals is extremely small.
- The linkage between Burkitt’s lymphoma and EBV infection is very high in the African endemic form of the disease and in cases of AIDS than in the sporadic form of the disease.
However, a few observations, especially regarding sporadic cases of Burkitt’s lymphoma, suggest that certain other supportive factors may be contributing. Immunosuppression appears to be one such significant factor.
The evidence in favour is as follows:
- Normal individuals harboring EBV infection as well as cases developing infectious mononucleosis are able to mount good immune response so that they do not develop Burkitt’s lymphoma.
- In immunosuppressed patients such as in HIV infection and organ transplant recipients, there is a marked reduction in the body’s T-cell immune response and a higher incidence of this neoplasm.
- It is observed that malaria, which confers immunosuppressive effects on the host, is prevalent in endemic proportions in regions where the endemic form of Burkitt’s lymphoma is frequent.
- This supports the linkage of EBV infection and immunosuppression in the etiology of Burkitt’s
lymphoma.

Anaplastic nasopharyngeal carcinoma:
This is the other tumor having the involvement of EBV infection. The tumor is prevalent in South-East Asia, especially in the Chinese, and in Eskimos.
The morphology of nasopharyngeal carcinoma. The evidence linking EBV infection with this tumor is as follows:
- 100% cases of nasopharyngeal carcinoma carry DNA of EBV in nuclei of tumor cells.
- Individuals with this tumor have high titers of antibodies to various EBV antigens.
However, like in case of Burkitt’s lymphoma, there may be some co-factors such as genetic susceptibility that account for the unusual geographic distribution.
EBV Oncogenesis:
The persistence of EBV infection is implicated in the etiology of malignancies of B lymphocytes and epithelial cells.
The mechanism of oncogenesis is as under :
- Latently infected epithelial cells or B lymphocytes express viral oncogene LMP1 (latent membrane protein) which is the most crucial step in the evolution of EBV-associated malignancies.
- Immunosuppressed individuals are unable to mount an attack against EBV infection and thus are more often affected.

- LMP1 viral protein dysregulates normal cell proliferation and survival of infected cells and acts like a CD40 receptor molecule on the B-cell surface.
- Thus, it stimulates B-cell proliferation by activating growth signaling pathways via nuclear factor κB (NF-κB) and JAK/STAT pathway.
- LMP-1 viral oncoprotein also activates BCL2 and thereby prevents apoptosis.
- Persistent EBV infection elaborates another viral protein EBNA-2 (EB virus nuclear antigen) which activates cyclin D and the SRC family of proto-oncogenes in the host cells and thus promotes cell proliferation.
- In immunocompetent individuals, LMP1 is kept under control by the body’s immune system.
Therefore, in these individuals lymphoma cells appear only after another characteristic mutation t(8;14) activates growth-promoting MYC oncogene.
Human Herpesvirus 8 (Hhv-8):
It has been shown that infection with HHV-8 is associated with Kaposi’s sarcoma, a vascular neoplasm common in patients of AIDS; hence also called Kaposi’s sarcoma-associated herpesvirus (KSHV).
Compared to sporadic Kaposi’s sarcoma, the AIDS-associated tumor is multicentric and more aggressive. HHV-8 has lymphotropism and is also implicated in the etiology of pleural effusion lymphoma and a multicentric variant of Castleman’s disease.
- HHV-8 (Kshv) Oncogenesis This is explained as under:
- Viral DNA is seen in the nuclei of all tumor cells in Kaposi’s sarcoma.
- There is overexpression of several KSHV oncoproteins by latently infected cells: v-cyclin, interferon regulatory factor (v-IRF), and LANA (latency-associated nuclear antigen).
- These viral proteins cause increased proliferation and survival of host cells and thus induce malignancy.
Adenoviruses
The human adenoviruses cause upper respiratory infections and pharyngitis.
- In humans, they are not known to be involved in any tumour.
- In hamsters, they may induce sarcomas.
Poxviruses
This group of oncogenic viruses is involved in the etiology of the following lesions:
- In rabbits—poxviruses cause myxomatosis.
- In humans—poxviruses cause molluscum contagiosum, a non-neoplastic skin lesion, and may induce squamous cell papilloma.
Hepadnaviruses
Hepatitis B virus (HBV) is a member of the hepadnavirus (HEPA- from hepatitis, -dna from DNA) family. HBV infection in man causes acute hepatitis and is responsible for a carrier state, which can result in some cases to chronic hepatitis progressing to hepatic cirrhosis, and onto hepatocellular carcinoma.
These lesions and the structure of HBV. There is strong epidemiological evidence linking HBV infection to development of hepatocellular carcinoma as observed from the following:
- The geographic distribution in the incidence of hepatocellular carcinoma close ly matches the variation in prevalence of HBV infection e.g. high incidence in Far-East and Africa.
- Epidemiological studies in high-prevalence regions indicate about 200 times higher risk of developing hepatocellular carcinoma in HBV-infected cases as compared to uninfected populations in the same area.
Those harbouring long-standing infection with HBV develops chronic destruction of HBV-infected hepatocytes followed by continued hepatocyte proliferation.
This process renders the hepatocytes vulnerable to the action of other risk factors such as to aflatoxin causing mutation and neoplastic proliferation.
Unlike HBV which is a DNA virus, an RNA hepatotropic virus, hepatitis C virus (HCV), in also implicated in the etiology of hepatocellular carcinoma. HCV is involved in about half the cases of hepatocellular carcinoma.
Hepatitis Virus Oncogenesis
Epidemiologic data firmly support that two hepatotropic viruses, HBV (a DNA virus) and HCV (an RNA virus), are currently involved in the etiology of 70- 80% of cases of hepatocellular carcinoma worldwide.
Although HBV DNA has been found integrated in the genome of human hepatocytes in many cases of liver cancer which causes mutational changes but a definite pattern is lacking. Thus, the exact molecular mechanism as to how HBV (as also HCV) cause hepatocellular carcinoma is yet not quite clear.
Probably, multiple factors are involved:
- Chronic and persistent viral infection with HBV (or HCV) incites repetitive cycles of inflammation, immune response, cell degeneration/cell death, and regeneration of the hepatocytes which leads to DNA damage of host liver cells.
- It is possible that the immune response by the host to persistent and unresolved infection with these hepatitis viruses becomes defective which promotes tumour development.
- On regeneration, a proliferation of hepatocytes is stimulated by several growth factors and cytokines elaborated by activated immune cells which contribute to tumor development for example,
Factors for angiogenesis, Cell survival, etc. - Activated immune cells produce nuclear factor κB (NF-kBκ) that inhibits apoptosis, thus allowing cell survival and growth.
- HBV genome contains a gene HBx which activates growth signaling pathway.
- HBV and HCV do not encode for any specific viral oncoproteins.
Leave a Reply