Flagellates
Giardia Lamblia (Intestinal Flagellates)
Habitat: Mucosa of duodenum and upper ileum of man.
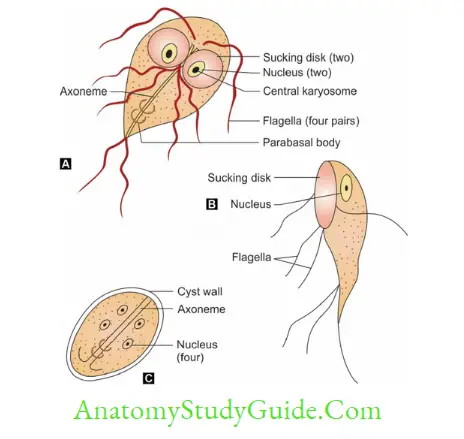
Morphology: 2 forms
Table of Contents
- Trophozoites
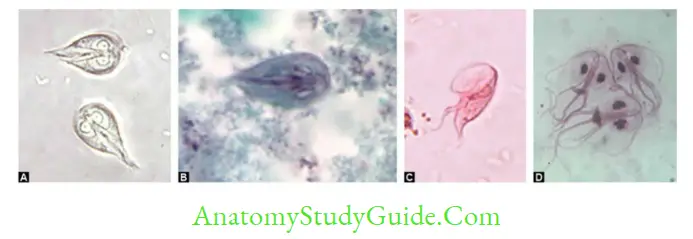
- Front view: Tear-drop shaped/racket shaped/piriform shaped, Lateral view: sickle/spoon-shaped
- Shows falling leaf-like motility, 10–20 μm in length and 5–15 μm in width.
- It has 2 nuclei, 4 pairs of flagella, 2 axonemes, 2 parabasal bodies, and two Ventral sucking disk
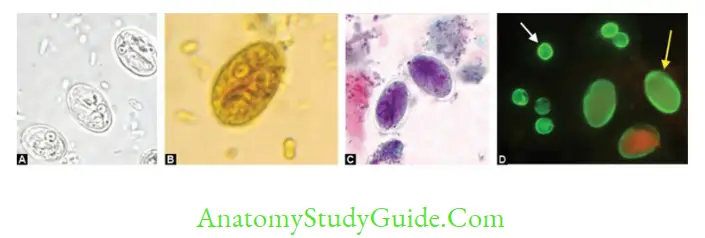
- Cyst: Mature cyst is oval, 11–14 μm in length and 7–10 μm in width, consisting of 4 nuclei and axonemes. Cysts are passed in feces.
Read And Learn More: Micro Biology And Immunology Notes
Life Cycle
- Infective stage: Cyst, Infective dose: As few as 10 to 25 cysts.
- Route of infection: Feco-oral route.
- Cyst transforms into trophozoites which multiply in the duodenum, attach to GIT mucosa by adhesive disk, and later on, shed in the lumen, transform to cysts which are passed in feces.
They are the diagnostic form of the parasite. - Risk factors: Children, elderly debilitated persons, and patients with cystic fibrosis, poor hygiene, reduced gastric acidity, prior gastric surgery, and immunodeficiency syndromes
(X-linked agammaglobulinemia). Not associated with HIV/AIDS.
Pathogenesis
- It causes abnormalities of villous structure and causes malabsorption (lipids and lipid-soluble vitamins)
- Malabsorption: There could be various types which include:
Malabsorption of fat (steatorrhea): Leads to foul-smelling profuse frothy diarrhea. - Disaccharidase deficiencies (lactate, xylose): Leading to lactose intolerance.
- Malabsorption of vitamin B12 and folic acid and protein loosing enteropathy.
- Antigenic variation in ‘variant surface protein’ of Giardia: results in chronic and persistent infection.
Clinical Features (Three Stages)
1. Asymptomatic carriers: Most infected persons are asymptomatic, harboring the cysts and spreading the infection.
2. Acute giardiasis:
- Incubation period 12–20 days.
- Common symptoms include diarrhea, abdominal pain, bloating, belching, flatus and vomiting
- Diarrhea is often foul smelling with fat, cellular exudate, and mucus but no blood
- The acute stage lasts for 1 week but usually resolves spontaneously
- Giardia is an important cause of traveler’s diarrhea.
3. Chronic giardiasis:
- Symptoms are intermittent and recurring
- Common symptoms-foul smelling diarrhea, foul flatus, sulfurous belching with rotten egg taste, and profound weight loss leading to growth retardation
- Extraintestinal manifestations such as urticaria, anterior uveitis, salt and pepper retinal changes, and arthritis.
Lab Diagnosis
- Samples collected: Stool sample and duodenal contents
- Microscopy by saline mount/iodine mount/trichome stain: Demonstrates either trophozoite (pear-shaped with falling leaf motility [in saline mount], indicates active infection) or cyst (indicates carrier/active infection)
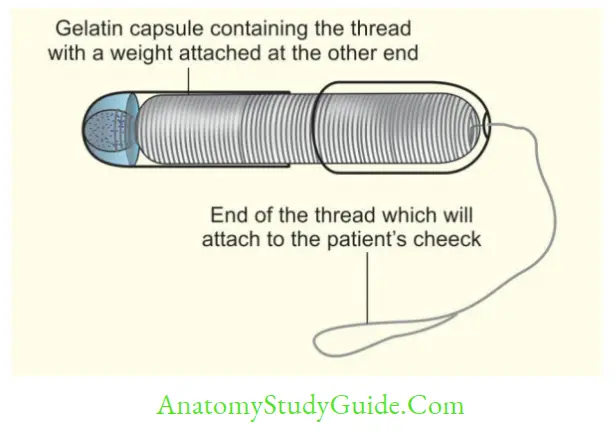
- String test/Entero test: Bile-stained mucus is collected for examination of Trophozoites
- Antigen detection in the stool (coproantigen) by ELISA or ICT (triage parasite panel that simultaneously detects antigens of Giardia, Entamoeba histolytica, and Cryptosporidium):
indicates active infection - Serum Antibody detection by ELISA, IFA: indicates past infection
- Molecular methods: BioFire FilmArray and molecular typing (by sequencing several genes; glutamate dehydrogenase (gdh), β-guardian (bg), and triosephosphate isomerase (tpi).
- Radiological finding: Barium meal X-ray
- Treatment: Metranidazole/Tinidazole, Nitazoxanide or Furazolidone.
Trichomonas Vaginalis(Genital Flagellates)
MC parasitic cause of STI (sexually transmitted infection) and NGU (nongonococcal urethritis)
Habitat: Urethra, vagina and prostate.
Morphology
- Trophozoite (flagellate and ameboid form) is the only form. No cystic stage. However, pseudocyst forms are seen in culture.
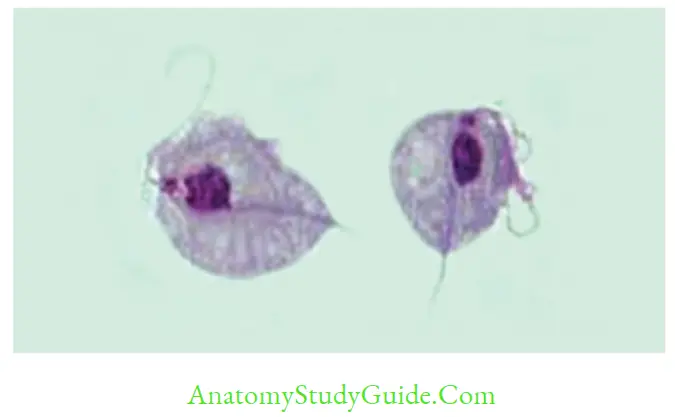
- Trophozoite: Shows twitching or jerky motility, pear-shaped consists of 5 flagella (4 anterior + 1 recurrent flagella) supported by an undulating membrane, a fibrillary structure called as costa and axostyle (runs down the middle of the trophozoite).
Life Cycle
- Mode of transmission: Sexual route
- Reservoir of infection: Woman
- Infective stage and diagnostic stage: Trophozoites
- Trophozoites divide by longitudinal binary fission.
Clinical Disease
- Incubation period: 4–28 days
- In men: Asymptomatic or Urethritis, Prostatitis and Cystitis
- In women: Asymptomatic or vulvovaginitis—Characterized by:
- Profuse vaginal discharge with offensive smell, high pH (> 4.5)
- Smell gets accentuated by adding 10% KOH (whiff test)
- Strawberry appearance of vaginal mucosa (Colpitis macularis)—seen in 2% of cases.
Lab Diagnosis
- Sample collected: Vaginal, and urethral discharges, Prostatic secretions or Urine sediment
- Microscopy of Vaginal, and urethral discharges: Actively motile (Jerky motility) trophozoites. Its sensitivity is (40–80%) and specificity is up to 100%
- Staining: Giemsa and Papanicolaou staining or Direct fluorescent antibody (DAF) test and acridine orange fluorescent stain
- Culture: More sensitive, gold standard e.g. Lash’s cysteine hydrolysate serum media
- Antigen detection in vaginal smear by ICT OSOM Trichomonas Rapid Test or ELISA: More sensitive than microscopy and indicates recent infection
- Serology by ELISA: Antibodies persist for long, so indicates past infection
- PCR detecting T. vaginalis specific beta-tubulin genes.
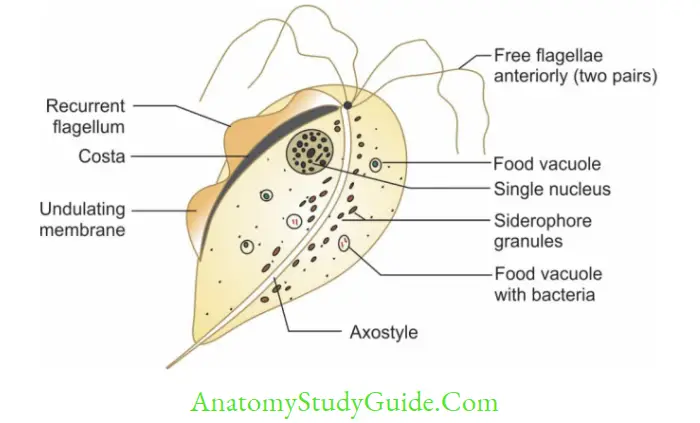
- Raised vaginal pH (>4.5)
- Whiff test (Fishy odor is accentuated when a drop of 10% KOH is added to vaginal discharge due to production of amine.
It is positive in more than 75% of cases. It is also positive in bacterial vaginosis.
Treatment
Drug of choice: Metronidazole or tinidazole (2 grams, single dose) to both sexual partners.
Resistance is rare but has been reported. If standard therapy fails, a second dose of metronidazole (2g) is given. and for Refractory cases (i.e. failure after two doses of standard therapy)—treatment with metronidazole 2 g for 5 days is recommended.
Hemoflagellates:(Leishmania And Trypanosoma)
- It exists in four morphological forms.
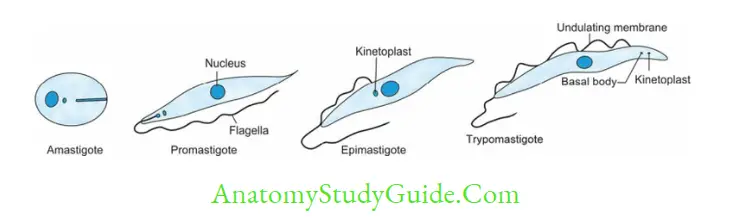
Leishmania
- Infective form: Promastigote
- Mode of transmission- By the bite of a female sandfly during the late evening or night time.
- A minimum of 10–1000 promastigotes per infective bite are required to initiate the infection.
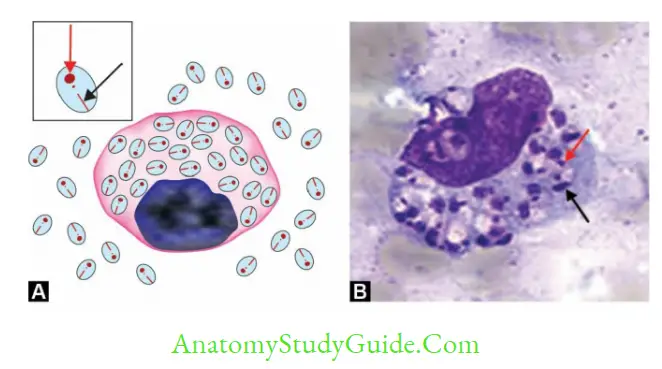
- Promastiogotes enter into skin macrophages and transform into amastigotes which are carried out in the circulation to various organs like liver, spleen, and bone marrow
- Diagnostic from in humans Amastigote form inside the macrophages- k/a LD body
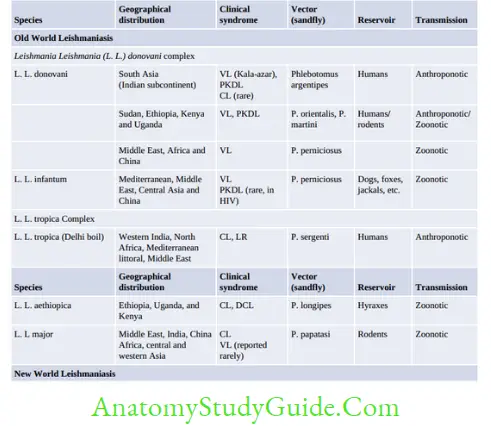
Epidemiology
- World: WHO estimated 7,00,000 to 1 million new cases and 20,000 to 30,000 deaths occur annually
- VL: An estimated 50,000 to 90,000 new cases of VL occur worldwide each year.
Over 90% of cases of VL come from three regions: (1) Southeast Asia: India, Bangladesh, and Nepal; (2) East Africa: Ethiopia, Sudan, and Kenya; and (3) Brazil - CL is the most common form of leishmaniasis with a global annual incidence of 6,00,000 to 1 million new cases.
About 95% of CL cases occur in the Americas, the Mediterranean basin, the Middle East, and Central Asia - MCL: Over 90% of mucocutaneous leishmaniasis cases occur in Bolivia, Brazil, Ethiopia, and Peru.
- VL: An estimated 50,000 to 90,000 new cases of VL occur worldwide each year.
- India: India is one of the worst affected countries.
- Bihar is affected the most (>70% of cases) followed by Jharkhand, West Bengal, and Uttar Pradesh.
- About 57 districts with more than 165 million of people are at risk. In 2017, about 5,758 cases of VL and 1,949 cases of PKDL were reported from India with nil death.
- VL reported maximum from Bihar (71.6%) and PKDL from Jharkhand (62%).
Kala-Azar
- Bone marrow involvement leads to:
- Anemia, leucopenia, thrombocytopenia
- Hypergammaglobulinemia
- Spleen ↑ , Liver ↑
- Fever and Hyperpigmentation (Indian cases)
- LN↑ (African but not in Indian cases)
- Kala-azar with HIV:
- Absence of hepatosplenomegaly
- GIT and resp. symptoms seen and relapses are common (CD4 T cell <100/mm3 favors the relapse)
- It is reported from Southern Europe (France, Italy, Spain, and Portugal) where 50–75% of adult cases of VL (usually caused by L. infantum) are HIV positive and 7–17% of HIV-infected people with fever have amastigotes. Also reported from other places like Africa (Ethiopia, Sudan), Brazil and India.
- In India, it is reported from Bihar, the sub-Himalayan region and other North Indian states.
Various studies reported a coinfection prevalence of 5–6% - Diagnosis: Amastigote detected from BAL and buffy coat region of blood. Antibodies are negative.
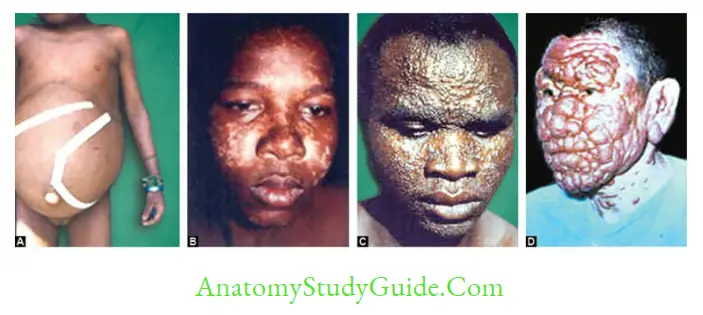
PKDL (Post Kala-azar Dermal Leishmaniasis)
- PKDL is a nonulcerative lesion of the skin that occurs in 2–50% of patients of VL following incomplete or inadequate treatment or treatment with antimonials.
- It is aggravated by exposure to sunlight. PKDL is also seen following L. infant infection, although rare.
- Mainly seen in India and East African countries.
- It develops as a hypopigmented macule (most common feature) near the mouth which later spreads to the face and then to arms and trunk (extensor surfaces) and finally becomes nodules resembling leprosy.
- Nodular lesions may serve as a reservoir of infection during the inter-epidemic period.
- Ocular lesions like conjunctivitis and uveitis are associated in some patients.
- The diagnosis is based on: Detection of amastigotes in the skin (in more than 80% of cases in Sudan) and serological tests such as Direct agglutination test (DAT) and antibodies to the rK39 antigen.
Virulence Factors
Glycoprotein (gp-63), Lipophosphoglycan (LPG), and Glycosylphosphatidylinositol.
Host Immune Response
- T helper 1 (Th1) Response:
- Protective response
- Leishmania skin test +ve
- T helper 2 (Th2) Response
- Indicates: Disease progression
- Leishmania skin test is –ve
Laboratory Diagnosis
- Clinical case definition: WHO has stated any case in an endemic area, with fever >2 weeks, splenomegaly, and/or weight loss is suspected of having VL and should be subjected to laboratory confirmation.
- Sample:
- Spleen (most Sensitive->98%)/Bone marrow aspiration (Commonly preferred sample,sensitivity-80–85%)
- Lymph node aspirate (Not useful in Indian cases)
- Blood: Buffy coat region and BAL (for HIV infected)
- Microscopy: Stained peripheral blood smear examination demonstrates LD bodies (amastigotes)
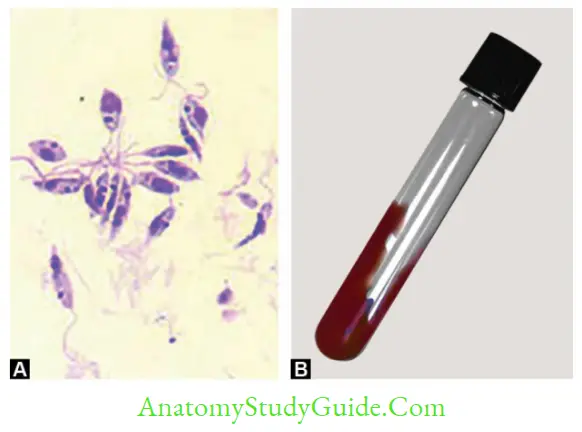
- Culture: NNN medium (McNeal, Novy and Nicolle) and Schneider’s Liquid medium (Amastigotes transform to promastigotes)
- Serological tests:
- Hypergammaglobulinemia: Detected by Napier’s aldehyde and Chopra’s antimony test
- CFT: Using tubercle bacilli antigen like WKK antigen
- Immunochromatographic test (ICT): Antibodies to rk39 or rKE16 antigens
- Direct agglutination test (DAT), ELISA, IFA
- Leishmania (Montenegro) skin test (indicates delayed hypersensitivity reaction):
- It is positive in people with good cell-mediated immunity, Observed in Patients with CL, LR, Recovered from VL
- However, this test is negative in (when CMI is low): Patients with active VL and DCL
- Molecular methods:
- Qualitative detection by PCR, nested PCR, and quantitative detection by real-time PCR are available targeting Leishmania-specific kinetoplast (mitochondrial) DNA; a sensitivity 70% to 93%.
- Other targets include 18S-rRNA, small subunit rRNA (more useful in HIV co-infection), and the gene encoding cysteine proteinase, β-tubulin, and gp63
- LAMP assay (loop-mediated isothermal amplification)
- Animal inoculation: Chinese and golden Hamsters.
Treatment
- Pentavalent antimonial: DOC in most endemic regions of the world, except in Bihar (increased resistance reported)
- Sodium stibogluconate
- Meglumine antimoniate
- Liposomal amphotericin B: DOC in Bihar
- Paromomycin – DIffuse CL (L. Ethiopia)
- Miltefosine.
- Pentamidine – DOC in CL due to L.guyanensis
- Prevention: Insect control. Sleeping on top floors also can prevent transmission.
Kala-Azar Elimination (NVBDCP)
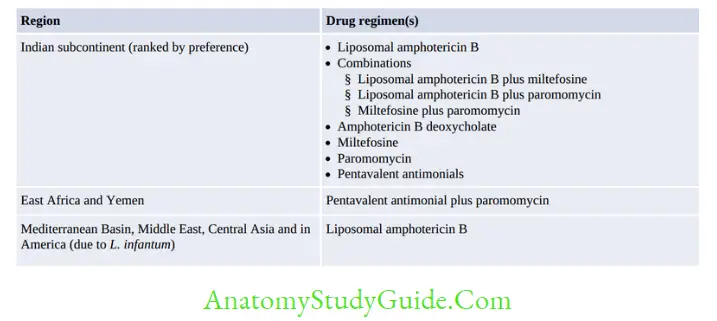
The National Vector-borne Disease Control Program (NVBDCP) launched an accelerated plan for kala-azar elimination in 2017.
The target for elimination is to reduce the annual incidence of kala-azar to less than one per 10,000 populations at the block PHC level.
The blocks in endemic areas of India are classified into four categories based on the annual incidence of kala-azar; each category has a specific action plan aiming toward kala-azar elimination, as described below

Trypanosoma cruzi
Life Cycle
- Mode of transmission:
- Rubbing of Reduviid bug following bite: Reduviid bugs are nocturnal in habitat and humans get an infection when abraded skin, mucous membranes, or conjunctivae become contaminated with reduviid bug’s feces containing an infective form of the parasite.
- By blood transfusion or organ transplantation,
- From mother to fetus
- Very rarely by ingestion of contaminated food or drink, and most importantly by laboratory accidents
- Infective form: Metacyclic Trypomastigotes
- Invades macrophages, and transforms to amastigotes which multiply in reticuloendothelial cells and other tissues like muscle (cardiac, skeletal, and GIT muscles) and nervous tissue.
- Amastigotes again transform to motile C-shaped non-multiplying trypomastigote forms which are released into blood following rupture of the host cells:
- Long slender forms are the invasive forms, migrate to many organs, and continue the life cycle
- Short stubby forms persist in the blood, to be taken up by the insect vector during a blood meal.

Acute Chagas’ Disease
- Chagoma (subcutaneous nodule)
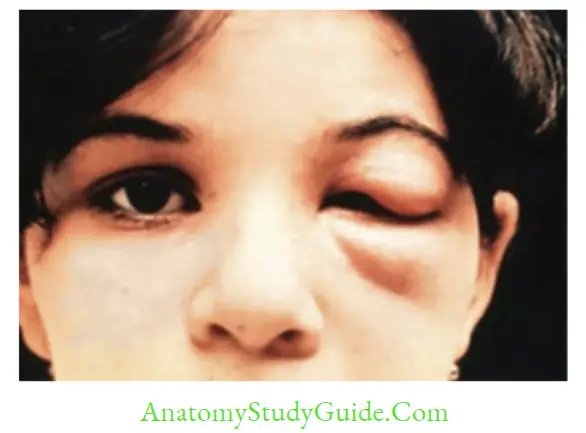
- Romana’s sign: Periorbital edema (observed in 48% of cases)
- Lymphadenopathy and hepatosplenomegaly.
Chronic Chagas’ Disease
- Autoimmune hypothesis: Occurs due to molecular mimicry
- Multiplication of the parasites in the muscles (skeletal, cardiac, and GIT) and CNS leads to:
- Cardiac form: Myocarditis and dilated cardiomyopathy
- Gastrointestinal form: Megaesophagus and megacolon
- Meningoencephalitis (↑ in HIV-infected people).
Congenital Trypanosomiasis
- Rarely, T. cruzi can be transmitted transplacentally both in the acute and chronic stages of the disease.
- It manifests as low birth weight, stillbirth, rarely myocarditis, and neurological alterations.
Association with HIV and HTLV-II
- HIV-infected people are at a greater risk of reactivation of underlying T. cruzi infection and are more prone to develop meningoencephalitis.
- Human T-lymphotropic virus 2 (HTLV-II)-infected people have a higher association with T.cruzi infection.
Diagnosis and Treatment
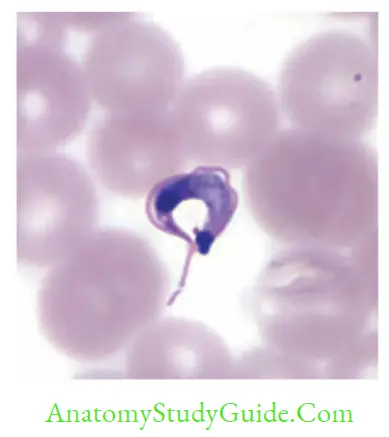
- Diagnostic form: C-shaped Trypomastigotes (20 μm) in blood smear. Often confused with T.rangeli (non-pathogen)
- Culture-Blood is inoculated in No media
- Antibody detection in serum: Radioimmune precipitation assay (Chagas’ RIPA- is highly sensitive & specific). Other methods include IFA, western blot, and enzyme strip assay
- Molecular methods—PCR, using 88 bp TCZ1–TCZ2 primers
- Antigen detection from serum, urine by CLIA (Chemiluminescent assay), and ELISA
- Xenodiagnosis—nymph of reduviid bugs
- Animal inoculation in mice.
Treatment: Benznidazole (DOC) and Nifurtimox.
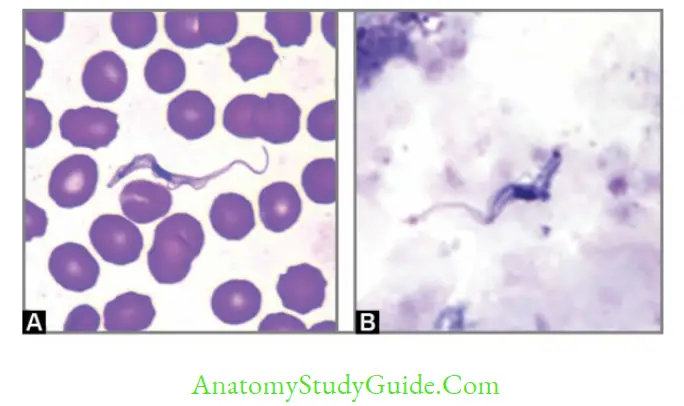
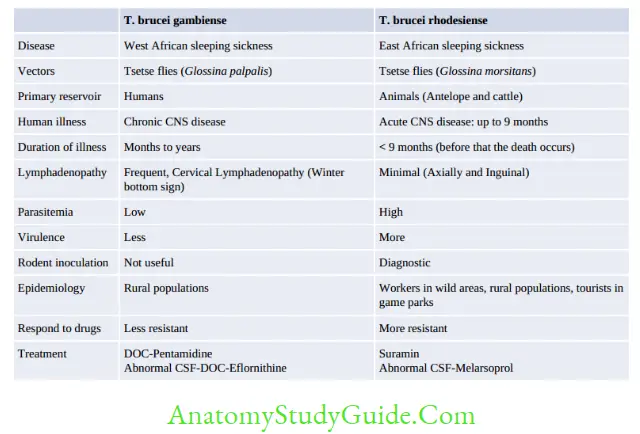
Trypanosoma Brucei
Life Cycle
- Infective form: Metacyclic trypomastigotes
- Mode of transmission: By bite of Tsetse fly
- Infective form: The metacyclic trypomastigote forms are found in the salivary gland of tsetse fly.
They transform into nondividing short stumpy form (15 μm long) without free flagellum; parasites invade the bloodstream resulting in parasitemia and migrate to various organs including the CNS. The short stumpy forms are the infective form to the tsetse fly - Undergo periodic antigenic variation of surface glycoprotein (VSG).
Clinical feature
- Trypanosomal chancre
- Stage I disease: Systemic febrile illness, lymphadenopathy (posterior cervical lymphadenopathy is called as Winterbottom’s sign)
- Stage II disease: Causes African sleeping sickness—Progressive daytime somnolence restlessness and insomnia at night.
Diagnosis
- Diagnostic form: Trypomastigotes seen in blood smear
- CSF shows Mott cells (abnormal plasma cells containing IgM)
- Antigens from serum and CSF: By ELISA and CIATT (The card indirect latex agglutination trypanosomiasis test): Useful for clinical staging of disease and for monitoring
- Antibody detection: Card agglutination test for trypanosomes (CATT), ELISA, and IFA.
- Molecular Methods: PCR, FISH (fluorescence in situ hybridization), branched DNA assay, real-time PCR, and LAMP (loop-mediated amplification technique).
Leave a Reply