Genetic Diseases
Human genetics refers to the study of individual genes and their functions in disease and their inheritance while genomics is the entire genetic information pertaining to the organism. Laid out broad principles of human genetics and genome. Here, the impact of errors in genes in causing human diseases is discussed.
Table of Contents
Read And Learn More: General Pathology Notes
- In the western countries, developmental and genetic birth defects constitute about 50% of total mortality in infancy and childhood,
- while in the developing and underdeveloped countries, 95% of infant mortality is attributed to environmental factors such as poor sanitation and undernutrition.
- Historically, genetic diseases have focused predominantly on developmental defects, chromosomal abnormalities, and disorders from single-gene defects.
Developmental Defects
Developmental defects are a group of abnormalities during foetal life due to errors in morphogenesis. The branch of science dealing with the study of developmental anomalies is called teratology.
- Certain chemicals, drugs, and physical and biological agents are known to induce such birth defects and are called teratogens.
- The morphologic abnormality or defect in an organ or anatomic region of the body so produced is called malformation.’
Pathogenesis Of Development Defects:
The teratogens may result in one of the following outcomes:
- Intrauterine death
- Intrauterine growth retardation (IUGR)
- Functional defects
- Malformation
The effects of teratogens in inducing developmental defects are related to the following factors:
- Variable individual susceptibility to teratogen: All patients exposed to the same teratogen do not develop birth defects.
- The intrauterine stage at which patient is exposed to teratogen: Most teratogens induce birth defects during the first trimester of pregnancy.
- Dose of teratogen: Higher the exposure dose of teratogen, the greater the chances of inducing birth defects.
- Specificity of developmental defect for specific teratogen: A particular teratogen acts in a particular way and induces the same specific developmental defect.
Classification Of Development Defects:
Various developmental anomalies resulting from teratogenic effects are categorised as under:
- Agenesis means the complete absence of an organ for example, Unilateral or bilateral agenesis of kidney.
- Aplasia is the absence of development of an organ with the presence of rudiment or anlage for example, Aplasia of lung with rudimentary bronchus.
- Hypoplasia is an incomplete development of an organ not reaching the normal adult size for example, Macroglossia.
- Atresia refers to incomplete formation of lumen in a hollow viscus for example , Oesophageal atresia.
- Developmental dysplasia is defective development of cells and tissues resulting in abnormal or primitive histogenetic structures for example, Renal dysplasia (Developmental dysplasia is quite different from cellular dysplasia in relation to precancerous lesions.
- Dysraphic anomalies are the defects resulting from the failure of fusion for example, Spina bifida. Ectopia or heterotopia refers to the abnormal location of tissue at ectopic site for example, Pancreatic heterotopia in the wall of the stomach.
Examples Of Developmental Defects:
A few clinically important examples are as under:
- Anencephaly-spina bifida complex: This is the group of anomalies resulting from failure to fuse (dysraphy). While anencephaly results from failure of neural tube closure, spina bifida occurs from incomplete closure of the spinal cord and vertebral column, often in the lumbar region. The latter results in meningocele or meningomyelocele.
- Thalidomide malformations: Thalidomide is the best known example of a teratogenic drug which was used as a sedative by pregnant women in 1960s in England and Germany and resulted in high incidence of limb-reduction anomalies (phocomelia) in newborns.
- Foetal hydantoin syndrome: Babies born to mothers on anti-epileptic treatment with hydantoin have characteristic facial features and congenital heart defects.
- Foetal alcohol syndrome: Ethanol is another potent teratogen. Consumption of alcohol by pregnant mothers in the first trimester increases the risk of miscarriages, stillbirths, growth retardation and mental retardation in the newborn.
- Torch complex Infection with TORCH group of organisms (Toxoplasma, Others, Rubella, Cytomegalovirus, and Herpes simplex) during pregnancy is associated with multisystem anomalies and TORCH syndrome in the newborn.
- Congenital syphilis: Vertical transmission of syphilis from mother to foetus is characterised by Hutchinson’s triad: interstitial keratitis, sensorineural deafness and deformed Hutchinson’s teeth, along with saddle-nose deformity.
Developmental Defects:
- Developmental defects are errors in morphogenesis during foetal life; these occur from use of teratogens during pregnancy.
- The effects of teratogens are intrauterine death and growth retardation, defects in functions or malformations.
- Developmental defects may be of varying grades: agenesis, aplasia, hypoplasia, atresia,
developmental dysplasia, failure of fusion and ectopia. - Some common examples are anencephaly, spina bifida, thalidomide malformations, foetal hydantoin and alcohol syndrome, TORCH complex, and lesions of congenital syphilis.
Cytogenetic (Karyotypic) Abnormalities
Human germ cells (ova and sperms) contain 23 chromosomes (haploid or 1N) while all the nucleated somatic cells of the human body contain 23 pairs of chromosomes (diploid or 2N)—44 autosomes and 2 sex chromosomes, being XX in females (46, XX) and XY in males (46, XY).
- The branch of science dealing with the study of human chromosomal abnormalities is called cytogenetics.
- In a female, one of the two X chromosomes (paternal or maternal derived) is inactivated during embryogenesis as stated in Lyon hypothesis.
- This inactivation is passed to all the somatic cells while the germ cells in the female remain unaffected i.e. ovary will always have an active X chromosome.
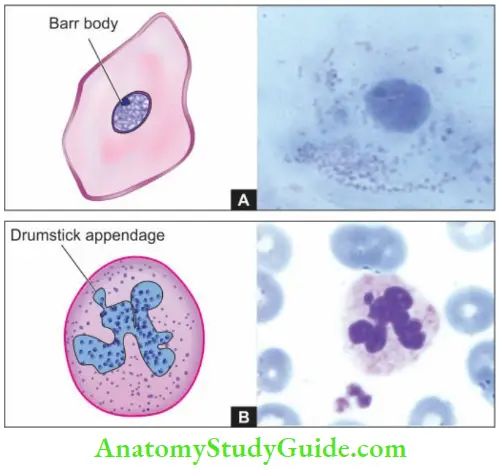
- Such an inactive X chromosome in the somatic cells in females lies condensed in the nucleus and is called as sex chromatin seen specifically in somatic cells in females.
- Nuclear sexing can be done for genetic female testing by preparing and staining the smears of squamous cells scraped from the oral cavity, or by identifying the Barr body in the circulating neutrophils as drumstick appendage attached to one of the nuclear lobes .’
- A minimum of 30% of cells positive for sex chromatin is indicative of genetically female composition. Though chromosomes can be studied in any human nucleated cells, circulating lymphocytes are more often used for this purpose.
- The study is done by arresting the dividing cells in metaphase by colchicine and then spreading them on glass slide and staining them with Giemsa stain. Karyotype is the photographic representation of the stained preparation of chromosomes.
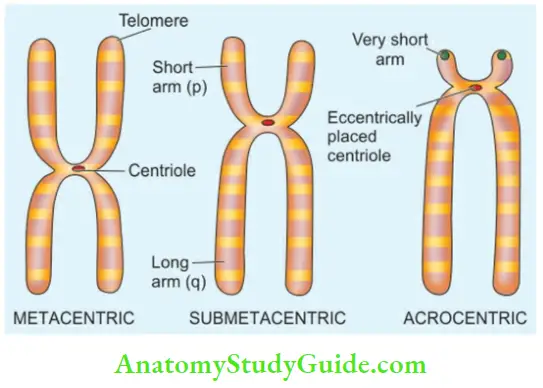
- The chromosomes are classified based on their length and location of the centromere; the centromere is the point where the two chromatids cross each other.
The distal end of each chromosome is called a telomere.
Based on centromeric location, they are classified into 3 groups:
- Metacentric chromosomes: (Numbers 1-3, 16, 19, 20) are those in which the centromere is exactly in the middle.
- Submetacentric chromosomes: (Numbers 4, 5, 17, 18, 6-12, and X) in which the centromere divides the chromosomes into short arm (p arm; petit means short in French) and long arm (q arm; for alphabet next to p).
- Acrocentric chromosomes: (Numbers 13, 14, 15, 21, 22, and Y) have very short arms and the centromere is eccentrically located.
Based on the length of chromosomes, they are divided into 7 groups A to G, called the Denver classification adopted at a meeting in Denver, Colorado in the US. Chromosomal banding techniques are employed for study of classes of chromosomes. Chromosomal bands are unique alternate dark and light staining patterns.
Banding techniques include:
- G-banding (Giemsa stain)
- Q-banding (quinacrine fluorescence stain)
- R-banding (reverse Giemsa staining), and
- C-banding (constitutive heterochromatin demonstration).
After these brief introductory comments, a discussion on abnormalities of chromosomes is done under two headings:
- Numerical abnormalities; and
- Structural abnormalities.
1. Numerical Abnormalities:
As mentioned above, the normal karyotype of a human nucleated somatic cell is diploid or 2N (46 chromosomes) while the germ cells have haploid or 1N (23 chromosomes).
- Polyploidy: Polyploidy is the term used for the number of chromosomes which is a multiple of haploid number for example Triploid or 3N (69 chromosomes), tetraploid or 4N (92 chromosomes).
Polyploidy occurs normally in megakaryocytes and dividing liver cells. Polyploidy in somatic cells of conceptus results in spontaneous abortions. - Aneuploidy: Aneuploidyis the number of chromosomes which is not an exact multiple of haploid numbers for example, Hypodiploid or 2N-1 (45 chromosomes) monosomy, hyperdiploid or 2 N+1 (47chromosomes) trisomy.
The most common mechanism of aneuploidy is nondisjunction. Nondisjunction is the failure of chromosomes to separate normally during cell division during first or second stage of meiosis, or in mitosis.
- Nondisjunction during the first meiotic division stage will result in two gametes from both the parental chromosomes due to failure to separate while the other two gametes will have no chromosomes (nullisomic).
- Nondisjunction during the second meiotic division stage results in one gamete with two identical
copies of the same chromosome, one nullisomic gamete, and two gametes with normal chromosome number. - Nondisjunction during mitosis results in mosaicism, meaning thereby that the individual has two or more types of cell lines derived from the same zygote. Mosaicism of mitotic nondisjunction of chromosomes occurs in cancers.
- Anaphase lag is a form of nondisjunction involving single pair of chromosomes in which one chromosome in meiosis or a chromatid in mitosis fails to reach the pole of the dividing cell at the same time (i.e. it lags behind) and is left out of the nucleus of the daughter cell. This results in one normal daughter cell and the other monosomic for the missing chromosome.
Three clinically important syndromes resulting from numerical aberrations of chromosomes due to nondisjunction are as under and their main clinical features are illustrated.
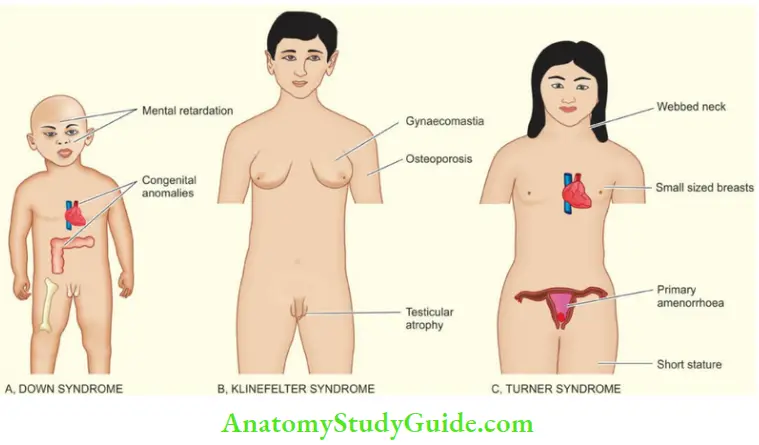
- Down syndrome: There is trisomy 21 in about 95% of cases of Down’s syndrome due to nondisjunction during meiosis in one of the parents. Down syndrome is the most common chromosomal disorder and is the commonest cause of mental retardation. The incidence of producing offspring with Down’s syndrome rises in mothers over 35 years of age.
- Klinefelter syndrome: Klinefelter syndrome is the most important example of sex chromosome trisomy. About 80% cases have 47, XXY karyotypes while others are mosaics.
- Typically, these patients have testicular dysgenesis. In general, sex chromosome trisomies are more common than trisomies of autosomes.
- Turner syndrome: Turner syndrome is an example of monosomy (45, X0) most often due to the loss of the X chromosome in paternal meiosis.
2. Structural Abnormalities
- During cell division (meiosis as well as mitosis), certain structural abnormalities of chromosomes may appear. These abnormalities may occur at different times as:
- During gametogenesis and then transmitted to all somatic cells and cause hereditary transmissible disorders, or May occur later as somatic cell mutations and result in changes that may range from ‘no effect’ to some forms of cancers.
Structural abnormalities may be balanced or unbalanced:
- Balanced structural alteration means no change in a total number of genes or genetic material.
- Unbalanced structural alteration refers to gene rearrangement resulting in the loss or gain of genetic material.
Some common forms of structural abnormalities are as under:
1. Translocations:
Translocation means the crossing over or exchange of fragment of chromosome which may occur between non-homologous or homologous chromosomes.
There are two main types of translocations: reciprocal in about two-thirds and Robertsonian in one-third of cases:
- Reciprocal translocation: Reciprocal translocation is the exchange of genetic material between two non-homologous (heterologous) chromosomes without involving centromere (acentric).
- Such translocations occur due to single breaks in both chromosomes and the exchange is detected by banding techniques.
- Reciprocal translocation may be balanced (without any loss of genetic material during the exchange) or unbalanced (with some loss of genetic material).
- Balanced reciprocal translocation is more common and the individual is phenotypically normal for example,
- Translocation between the long arm (q) of chromosome 22 and the long arm (q) of chromosome 9 is written as t (9;22). This translocation is termed the Philadelphia chromosome seen in most cases of chronic myeloid leukaemia.
- Unbalanced reciprocal translocations are less common and account for repeated abortions and malformed children.
- Robertsonian translocation: Robertsonian translocation is less common than reciprocal translocation. In this, there is a fusion of two acrocentric chromosomes (having very short arms) at the centromere (centric fusion) with the loss of short arms.
The result of this fusion is one very large chromosome and the other very small one. Individuals born with Robertsonian translocation may be phenotypically normal but suffer from infertility and are at higher risk of producing malformed children in the next progeny.
2. Deletions:
Loss of genetic material from the chromosome is called deletion. Deletion may be from the terminal or middle portion of the chromosome.
Examples of deletion are: Cri du chat (cry of infant like that of a cat) syndrome (deletion of the short arm of chromosome 5) and several cancers with hereditary basis (for example Retinoblastoma with deletion of long arm of chromosome 13, Wilms’ tumour with deletion of short arm of chromosome 11).
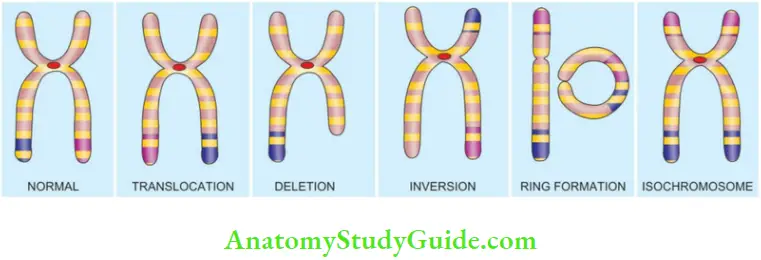
3. Inversion:
An inversion is a form of rearrangement involving breaks of a single chromosome at two points. Inversion may be pericentric or paracentric, depending upon whether the rotation occurs at the centromere or at the acentric portion of the arm of the chromosome. Inversions are not associated with any abnormality.
4. Ring Chromosome:
A ring of the chromosome is formed by a break at both the telomeric (terminal) ends of a chromosome followed by deletion of the broken fragment and then end-to-end fusion. The consequences of ring chromosomes depend upon the amount of genetic material lost due to break.
5. Isochromosome:
When the centromere, rather than dividing parallel to the long axis, instead divides transverse to the long axis of the chromosome, it results in either two short arms only or two long arms only called isochromosomes. The example involving the isochromosome of Xchromosome is seen in some cases (15%) of Turner’s syndrome.
Cytogenetic (Karyotypic) Abnormalities:
Abnormalities of chromosomes may be numerical or structural. Numerical abnormalities may be due to polyploidy (i.e. multiple of haploid number) or aneuploidy (i.e. which is not a multiple of haploid number).
- A few common examples of numerical aberrations of chromosomes due to nondisjunction are Down syndrome (trisomy 21), Klinefelter syndrome (47, XXY) and Turner syndrome (45, XO).
- Common structural abnormalities are translocations (reciprocal or Robertsonian) and deletions; others are inversion, ring chromosomes, and isochromosomes.
Mendelian Disorders
The classic laws of inheritance of characteristics or traits were outlined by Austrian monk Gregor Mendel in 1866 based on his observations of cross-breeding of red and white garden peas.
- Single-gene defects follow the classic Mendelian patterns of inheritance and are also called Mendelian disorders. These disorders are the result of mutation of a single gene of large effect.
- On the other hand, some normal phenotypic characteristics have multifactorial inheritance, for example, Colour of hair, eye, skin, height and intelligence.
- Multifactorial disorders are those disorders which result from the combined effect of genetic composition and environmental influences.
Some common examples of such disorders in which environmental influences unmask and express the mutant genes are as under:
- Cleft lip and cleft palate
- Pyloric stenosis
- Diabetes mellitus
- Hypertension
- Congenital heart disease
- Coronary heart disease
Mutations:
The term mutation is applied to permanent change in the DNA of the cell. Mutations affecting germ cells are transmitted to the next progeny producing inherited diseases, while the mutations affecting somatic cells give rise to various cancers and congenital malformations.
Presently, the following types of mutations have been described:
- Point mutation: Point mutation is the result of the substitution of a single nucleotide base by a different base i.e. replacement of an amino acid by another for example, in Sickle cell anaemia there is point mutation by substitution of glutamic acid by valine in the polypeptide chain.
- Stop codon or nonsense mutation: Stop codon or nonsense mutation refers to a type of mutation in which the protein chain is prematurely terminated or truncated.
- Frameshift mutation: Frameshift mutation occurs when there is insertion or deletion of one or two base pairs in the DNA sequence for example, Cystic fibrosis of the pancreas.
- Trinucleotide repeat mutation: Trinucleotide repeat mutation is characterised by the amplification of a sequence of three nucleotides.
Thus, it can be summed up from above that single-gene defects are synonymous with various types of heritable mutations. Currently, approximately 5000 single-gene defects have been described—some major and others of minor consequence.
While most of these disorders are discussed in relevant chapters later, the group of storage diseases (inborn errors of metabolism) is described here.
Inheritance Pattern:
The inheritance pattern of genetic abnormalities may be dominant or recessive, autosomal or sex-linked:
A dominant gene produces its effects, whether combined with a similar dominant or recessive gene. Recessive genes are effective only if both genes are similar. However, when both alleles of a gene pair are expressed in the heterozygous state, it is called codominant inheritance.
A single gene may express in multiple allelic forms known as polymorphism. Autosomal diseases are due to defects in any of 1–22 autosomes while sex-linked disorders are mostly X-linked.
- Autosomal dominant inheritance: Autosomal dominant inheritance pattern is characterised by one faulty copy of the gene (i.e. mutant allele) in any autosome and one copy of a normal allele; disease phenotype is seen in all such individuals.
- Patients having autosomal dominant inheritance disease have 50% chance of passing on the disease to the next generation.
- In autosomal recessive inheritance: In autosomal recessive inheritance Both copies of genes are mutated. Usually, it occurs when both parents are carriers of the defective gene, i.e. having one normal allele and one defective allele in each parent, and each parent passes on their defective gene to the next progeny causing disease.
- There is a 25% chance of transmission of autosomal recessive disease when both parents are carriers.
- X-linked disorders: X-linked disorders are caused by mutations in genes on the X-chromosome, derived from either one of the two X-chromosomes in females or from the single X-chromosome of the male.
- There are much fewer genes on the Y-chromosome and are determinants for testis. Y-linked diseases are rare and include male infertility, excessive hair on the pinna, retinitis pigmentosa, and XYY syndrome.
- Inheritance pattern-based list of common Mendelian disorders due to single-gene defect is given in Table
Inheritance patterns of these disorders may be: Autosomal recessive (the largest group), codominant (intermediate), and dominant, and sex-(X-) linked recessive and dominant disorders. A few selected examples of conditions are given below while others are discussed in realted chapters of Systemic Pathology.
Disorders Due To Protein Defects
Depending upon the level and type of protein defect, these disorders may be of 3 types:
- Structural protein disorders
- Receptor protein disorders, and
- Growth regulatory protein disorders.
1. Structural Protein Disorders:
These are conditions due to mutations in genes coding for structural proteins which may be extracellular matrix (ECM) proteins or cell membrane proteins.
- ECM protein disorders: These are due to genetic defects in genes encoding extracellular matrix (ECM) proteins, collagen or fibrillin.
- Defect in collagen is due to deletion or point mutation that results in either reduced production of normal collagen or a normal amount of abnormal collagen.
- Examples of disorders due to genetic defects in collagen are, Osteogenesis imperfecta and Ehlers-Danlos syndrome. Due to defects in collagen,
- Ehlers-Danlos syndrome presents with abnormalities in collagen-rich tissues such as joints (hypermobility), skin (hyperextensibility), and internal tissues (for example, Lack of tensile strength in the colon, large blood vessels etc).
- Defect in fibrillin is due to a missense mutation in the FBN1 gene that codes for fibrillin; for example, Marfan Syndrome. In this condition, there is lack of structural rigidity due to genetic defects in ECM protein fibrillin.
- Clinically, patients of Marfan syndrome have skeletal manifestations (tall in height, long fingers), ocular lesions (subluxation of the lens), and cardiovascular manifestations (mitral valve prolapse, aortic aneurysm, and aortic dissection).
Cell membrane protein: Cell membrane protein disorders Genes coding for membrane proteins are dystrophin, spectrin and ankyrin.
Genetic mutations in these proteins result in following disorders:
-
- Defect in dystrophin due to deletion causes muscular dystrophy (Duchenne or Becker type).
- Defects in spectrin and ankyrin result in hereditary spherocytosis.
Important examples of Mendelian disiorders

2. Receptor Protein Disorders:
These disorders are due to genetic mutation in receptor proteins, LDL (low-density lipoprotein) receptor and vitamin D receptor:
- LDL receptor defect: Classic example of a disorder due to mutation of gene coding for LDL receptor is familial hypercholesterolaemia.
- This is an autosomal dominant condition in which patients develop hypercholesterolaemia from reduced transport of LDL into the cells.
- Patients have high blood lipids at an early age, and develop atherosclerosis and coronary artery disease at young age.
- Vitamin D receptor defect: A mutation in this receptor causes failure of normal signalling of vitamin D and results in vitamin D-resistant rickets.
3. Growth Regulatory Protein Disorders:
As gene products of major growth regulatory genes, oncogenes and tumour-suppressor genes, maintain a balance for normal cell growth and differentiation. Mutations in these regulatory genes is the main molecular basis of most tumours.
For example inherited retinoblastoma from a mutated RB gene, neurofibromatosis due to a mutated NF gene etc.
Storage Disorders (Inborn Errors Of Metabolism)
Storage diseases or inborn errors of metabolism are biochemically distinct groups of disorders occurring due to genetic defects in the metabolism of carbohydrates, lipids, and proteins resulting in intracellular accumulation of metabolites.
- These substances may collect within the cells throughout the body but most commonly affected organ or site is the one where the stored material is normally found and degraded.
- Since lysosomes comprise the chief site of intracellular digestion (autophagy as well as heterophagy), the material is naturally stored in the lysosomes, hence the generic name ‘lysosomal storage diseases’.
- Cells of mononuclear-phagocyte system are particularly rich in lysosomes; therefore, reticuloendothelial organs containing numerous phagocytic cells like the liver and spleen are most commonly involved in storage disease.
- Based on the biochemical composition of the accumulated material within the cells, storage diseases are classified into distinct groups, each group containing a number of diseases depending upon the specific enzyme deficiency.
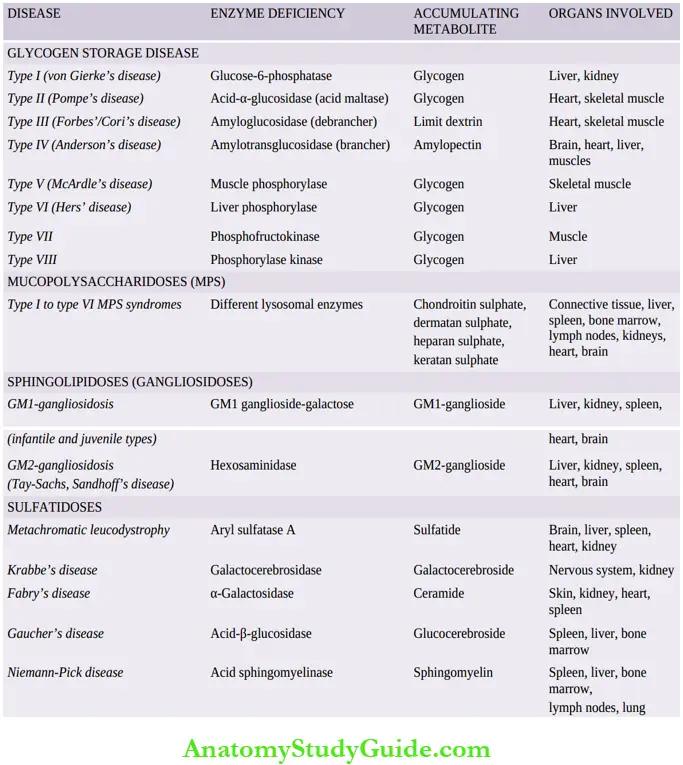
- A summary of major groups of storage diseases along with their respective enzyme deficiencies, major accumulating metabolites and the organs.
A few general comments can be made about all storage diseases:
- All the storage diseases occur either as a result of autosomal recessive, or sex-(X-) linked recessive genetic transmission.
- Most, but not all, of the storage diseases are lysosomal storage diseases. However, out of the glycogen storage diseases, type II (Pompe’s disease) is the only example of a lysosomal storage disease.
A few important forms of storage diseases are described below:
1. Glycogen Storage Diseases (Glycogenoses):
These are a group of inherited disorders in which there is defective glucose metabolism resulting in excessive intracellular accumulation of glycogen in various tissues.
Based on specific enzyme deficiencies, glycogen storage diseases are divided into 8 main types designated by Roman numerals I to VIII.
However, based on pathophysiology, glycogen storage diseases can be divided into 3 main subgroups:
- Hepatic forms: Hepatic forms are characterised by an inherited deficiency of hepatic enzymes required for synthesis of glycogen for storage (for example, Von Gierke’s disease or type I glycogenosis) or due to lack of hepatic enzymes necessary for breakdown of glycogen into glucose (for example,Type VI glycogenosis).
- Myopathic: Myopathic forms on the other hand, are those disorders in which there is a genetic deficiency of glycolysis to form lactate in the striated muscle resulting in accumulation of glycogen in the muscles (for example, McArdle’s disease or type V glycogenosis, type VII disease).
- Other: Other forms are those in which glycogen storage does not occur by either hepatic or myopathic mechanisms. In Pompe’s disease or type II glycogenosis, there is lysosomal storage of glycogen, while in type IV there is the deposition of abnormal metabolites of glycogen in the brain, heart, liver and muscles
Storage diseases (inborn errors of metabolism):
The prototypes of these three forms are briefly considered below.
- Von Gierke’S Disease (Type I Glycogenosis):
- This condition is inherited as an autosomal recessive disorder due to a deficiency of the enzyme, glucose-6-phosphatase.
- In the absence of glucose-6-phosphatase, excess of the normal type of glycogen accumulates in the liver and also results in hypoglycaemia due to reduced formation of free glucose from glycogen.
- As a result, fat is metabolised for energy requirements leading to hyperlipoproteinaemia and ketosis.
- Other changes due to deranged glucose metabolism are hyperuricaemia and accumulation of pyruvate and lactate.
- The disease manifests clinically in infancy with failure to thrive and stunted growth.
- A most prominent feature is enormous hepatomegaly with intracytoplasmic and intranuclear glycogen.
- The kidneys are also enlarged and show intracytoplasmic glycogen in tubular epithelial cells.
- Other features include gout, cutaneous xanthomas and bleeding tendencies due to platelet dysfunction.
- Pompe’S Disease (Type II Glycogenosis):
- This is also an autosomal recessive disorder due to a deficiency of a lysosomal enzyme, acid maltase, and is the only example of lysosomal storage disease amongst the various types of glycogenosis.
- Acid maltase is normally present in most cell types and is responsible for the degradation of glycogen.
- Its deficiency, therefore, results in the accumulation of glycogen in many tissues, most often in the heart and skeletal muscle, leading to cardiomegaly and hypotonia.
- Mcardle’S Disease (Type V Glycogenosis):
- The condition occurs due to a deficiency of muscle phosphorylase resulting in the accumulation of glycogen in the muscle (deficiency of liver phosphorylase results in type VI glycogenosis).
- The disease is common in 2nd to 4th decades of life and is characterised by painful muscle cramps, especially after exercise, and the detection of myoglobinuria in half the cases.
2. Mucopolysaccharidoses (MPS):
Mucopolysaccharidoses are a group of six inherited syndromes numbered from MPS I to MPS
Each of these result from a deficiency of a specific lysosomal enzyme involved in the degradation of mucopolysaccharides or glycosaminoglycans, and are, therefore, a form of lysosomal storage disease.
Mucopolysaccharides which accumulate in the MPS are:
- chondroitin sulphate, dermatan sulphate, heparan sulphate and keratan sulphate.
- All these syndromes are autosomal recessive disorders except MPS II (Hunter’s syndrome) which has X-linked recessive transmission.
- Syndrome of MPS manifests in infancy or early childhood and involves multiple organs and tissues, chiefly connective tissues, liver, spleen, bone marrow, lymph nodes, kidneys, heart and
brain. - The mucopolysaccharides accumulate in mononuclear phagocytic cells, endothelial cells, intimal smooth muscle cells and fibroblasts.
- The material is finely granular and PAS-positive by light microscopy.
- By electron microscopy, it appears in the swollen lysosomes and can be identified biochemically as a mucopolysaccharide.
3. Gaucher’s Disease:
This is an autosomal recessive disorder in which there is a mutation in the lysosomal enzyme, acid β- glucosidase (earlier called glucocerebrosidase), which normally cleaves glucose from ceramide.
- This results in lysosomal accumulation of glucocerebroside (ceramide-glucose) in phagocytic cells of the body and sometimes in the neurons.
- The main sources of glucocerebroside in phagocytic cells are the membrane glycolipids of old leucocytes and erythrocytes, while the deposits in the neurons consist of gangliosides.
Clinically, 3 subtypes of Gaucher’s disease are identified based on neuronopathic involvement:
- Type I or the classic form is the adult form of the disease in which there is the storage of glucocerebrosides in the phagocytic cells of the body, principally involving the spleen, liver, bone marrow, and lymph nodes. Thus, this is the non-neuronopathic type and is the most common type comprising 80% of all cases of Gaucher’s disease.
- Type II is the infantile form in which there is progressive involvement of the central nervous system.
- Type III is the juvenile form of the disease having features in between type I and type II i.e. they have systemic involvement like in type I and progressive involvement of the CNS as in type II.
Clinical features depend upon the clinical subtype of Gaucher’s disease. In addition to the involvement of different organs and systems (splenomegaly, hepatomegaly, lymphadenopathy, bone marrow and cerebral involvement), a few other features include pancytopenia, or thrombocytopenia secondary to hypersplenism, bone pains and pathologic fractures.
Microscopy:
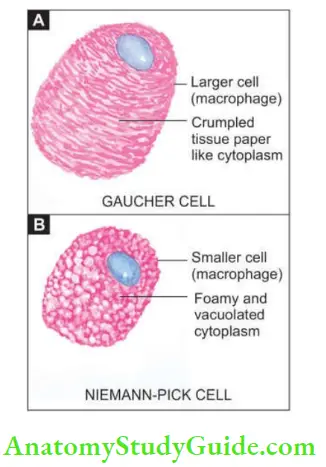
Shows large number of characteristically distended and enlarged macrophages called Gaucher cells which are found in the spleen, liver, bone marrow and lymph nodes, and in the case of neuronal involvement, in the Virchow-Robin space.
- The cytoplasm of these cells is abundant, granular and fibrillar resembling crumpled tissue paper. They have mostly a single nucleus but occasionally may have two or three nuclei
- Gaucher cells are positive with PAS, oil red O, and Prussian-blue reaction indicating the nature of accumulated material as glycolipids admixed with haemosiderin. These cells often show erythrophagocytosis and are rich in acid phosphatase.
4. Niemann-Pick Disease:
This is also an autosomal recessive disorder characterised by the accumulation of sphingomyelin and cholesterol due to a defect in acid sphingomyelinase.
Two types have been described: types A and B.
- Type A: Type A is more common and typically presents in infancy and is characterised by hepatosplenomegaly, lymphadenopathy, rapidly progressive deterioration of CNS and physical underdevelopment.
- About a quarter of patients present with familial amaurotic idiocy with characteristic cherry-red spots in the macula of the retina (amaurosis = loss of vision without apparent lesion of the eye).
- Type B: Type B develops later and has a progressive hepatosplenomegaly with the development of cirrhosis due to replacement of the liver by foam cells, and impaired lung function due to infiltration in lung alveoli.
Microscopy:
Shows storage of sphingomyelin and cholesterol within the lysosomes, particularly in the cells of the mononuclear phagocyte system.
- The cells of Niemann-Pick disease are somewhat smaller than Gaucher cells and their cytoplasm is not wrinkled but is instead foamy and vacuolated which stains positively with fat stains.
- These cells are widely distributed in the spleen, liver, lymph nodes, bone marrow, lungs, bowel and brain.
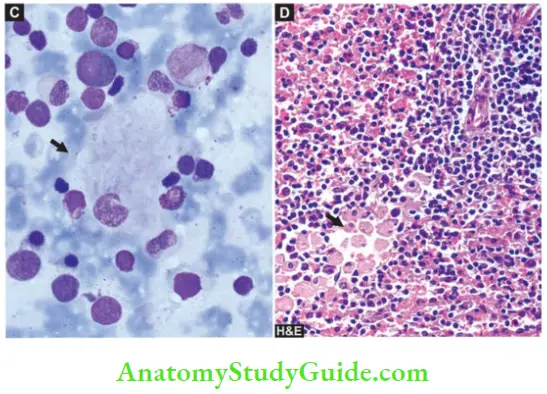
- Diagrammatic view of comparative features of typical Gaucher cell (A) and typical macrophage in Niemann-Pick disease (B). C, Gaucher cell (arrow) in bone marrow aspirate smear. D, Infiltration by Gaucher cells in red pulp of splenic parenchyma.
Mendelian Disorders (Single Gene Defects):
The mutation is a permanent change in the DNA of the cell. Mutations are of different types: Point, Stop codon (nonsense), Frameshift and Trinucleotide repeat.
- Inheritance patterns of genetic abnormalities may be dominant or recessive, autosomal or sex-linked. Most sexlinked diseases are actually X-linked since Y chromosome has very few functional genes.
- Multifactorial inheritance is responsible for several normal phenotypic characters. Hypertension and pyloric stenosis are common examples.
- Genetic disorders due to defects in protein may be due to 1) defects in structural proteins (ECM protein defects in collagen for example Ehlers-Danlos syndrome, osteogenesis imperfect; defects in fibrillin Marfan syndrome; cell membrane defects for example, Hereditary spherocytosis, muscular dystrophy),
- For example, receptor protein disorders (for example, Familial Hypercholesterolaemia, vitamin D-resistant rickets), and
- Growth regulatory protein disorders (for example, Various tumours due to mutations in oncogenes and tumour-suppressor genes).
- Storage diseases or inborn errors of metabolism are biochemically distinct disorders occurring due to genetic defects in the metabolism of carbohydrates, lipids, and proteins resulting in intracellular accumulation of metabolites.
- Most of storage diseases are lysosomal storage diseases. Out of the glycogen storage diseases, type II (Pompe’s disease) is the only example of lysosomal storage disease.
- Mucopolysaccharidoses are inherited syndromes resulting from a deficiency of specific lysosomal enzymes involved in the degradation of mucopolysaccharides or glycosaminoglycans.
- Gaucher’s disease is an autosomal recessive disorder due to a mutation in the lysosomal enzyme, acid ß-glucosidase (earlier called glucocerebrosidase), which normally cleaves glucose from ceramide.
- The Niemann-Pick disease is also an autosomal recessive disorder characterised by the accumulation of sphingomyelin and cholesterol due to a defect in acid sphingomyelinase.
Leave a Reply