Irrigation And Intracanal Medicaments Notes
Irrigation And Intracanal Medicaments
Successful endodontic treatment depends on a combination of proper instrumentation, irrigation, and three-dimensional obturation of the root canal system. Among these, irrigation plays an indispensable role in endodontic treatment.
Table of Contents
It is truly said, “Instruments shape, irrigants clean.” Irrigation is the only way to clean those areas of root canal walls that are not touched by mechanical instrumentation. These areas are fis, isthmuses, anastomosis, and large lateral canals.
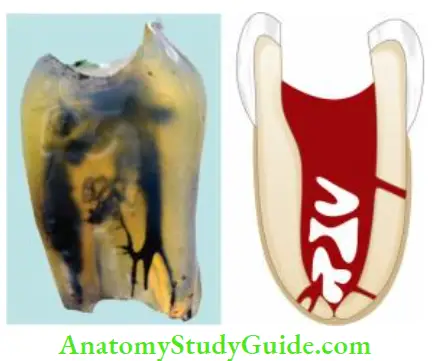
The objective of using an irrigant is chemical dissolution/disruption, mechanical detachment, removal of the pulp tissue, dentin debris, and smear layer, microorganisms, and their products out of the root canal system.
Read And Learn More: Endodontics Notes
Ideal Requirements For An Irrigant
It should
- Have broad-spectrum antimicrobial properties
- Aid in the debridement of the canal system
- Have the ability to dissolve necrotic tissue or debris
- Have low toxicity level
- Be a good lubricant
- Have low surface tension so that it can easily flow into inaccessible areas
- Be able to effectively sterilize the root canal (or at least disinfect them)
- Be able to prevent the formation of a smear layer during instrumentation or dissolve the latter once it is formed
- Inactivate endotoxin
Other desirable properties of an ideal irrigant are that it should
- Be able to penetrate root canal periphery
- Be able to dissolve pulp tissue, smear layer, and biofilm
- Be bactericidal even for microorganisms in biofilm
- Be fungicidal
- Not weaken the tooth structure
- Be easily available
- Be cost-effctive
- Be easy to use
- Have an adequate shelf life
- In addition to these properties, if endodontic irrigants come in contact with vital tissue, these should be sys- chemically nontoxic, noncaustic to the periodontal tissue, and have little potential to cause an anaphylactic reaction
Functions Of Irrigants
- Irrigants perform physical and biological functions. They remove dentin shavings from canals and thus prevent blockage of canal apex
- The effiency of instruments increases in wet canals and they are less likely to break in lubricated canals
- Irrigants act as a solvent of necrotic tissue, so they loosen debris, pulp tissue, and microorganisms from irregular dentinal walls
- Irrigants help in remove the debris from fis, – miosis, accessory, and lateral canals where instruments cannot reach
- Most irrigants are germicidal and have antibacterial action
- The bleaching action of irrigants lightens the teeth discolored due to trauma or extensive silver restorations
Factors That Modify the Activity Of Irrigating Solutions
Efficy of irrigating solutions depends on the following factors:
Concentration:
Studies have shown that though the tissue-dissolving ability of sodium hypochlorite increases linearly with concentration, it is preferred to use a lower concentration due to its cytotoxic effects.
Contact:
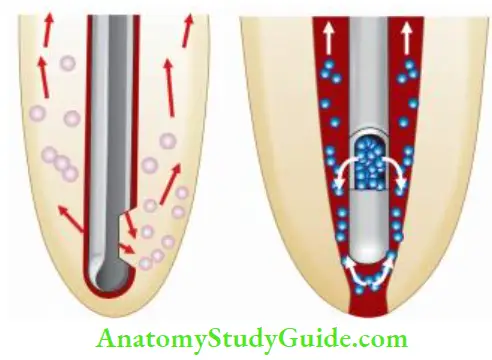
To be effective, the irrigant must contact the substrate (organic tissue or microbes). When canals are sufficiently enlarged, irrigant can be deposited directly in the apical area with a fie irrigating needle to increase its effect.
Presence of Organic Tissue:
The presence of organic tissues decreases the effctiveness of intracanal medicaments. The protein content of organic tissues form a coagulate when reacts with medicament. This coagulant serves as a barrier to prevent further penetration of medicament, thus limiting its effctiveness.
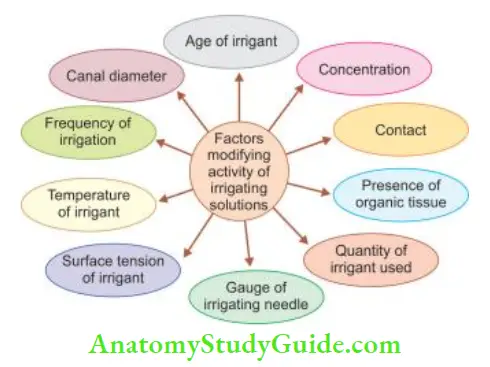
Quantity of Irrigant Used:
The quantity of irrigant used is directly related to its ability to remove the debris from the canal.
The gauge of Irrigating Needle:
27- or 28-gauge needle is preferred as it can go deeper in the canal for better delivery and debridement action.
Surface Tension of Irrigant:
The lower surface tension of an irrigant increases its wettability, hence better penetration in narrow areas for effctive debridement.
Temperature of Irrigant:
As the temperature of the irrigant is increased, its efficacy increases.
Frequency of Irrigation:
Copious irrigation has the following advantages:
- More irrigation causes better debridement of tissues
- Each time a fresh potent irrigant plays an action
Canal Diameter:
The wider the canal, the better is the debridement action of the irrigant.
Age of Irrigant;
Freshly prepared solutions are more efficient, than older ones.
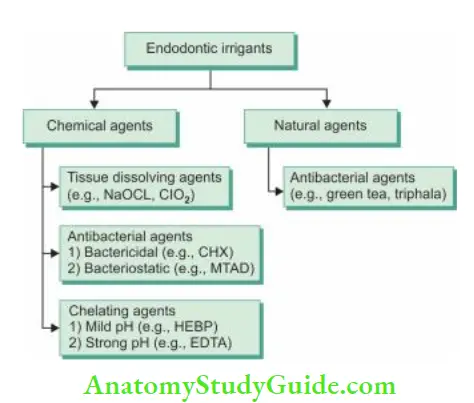
Commonly Used Irrigating Solutions
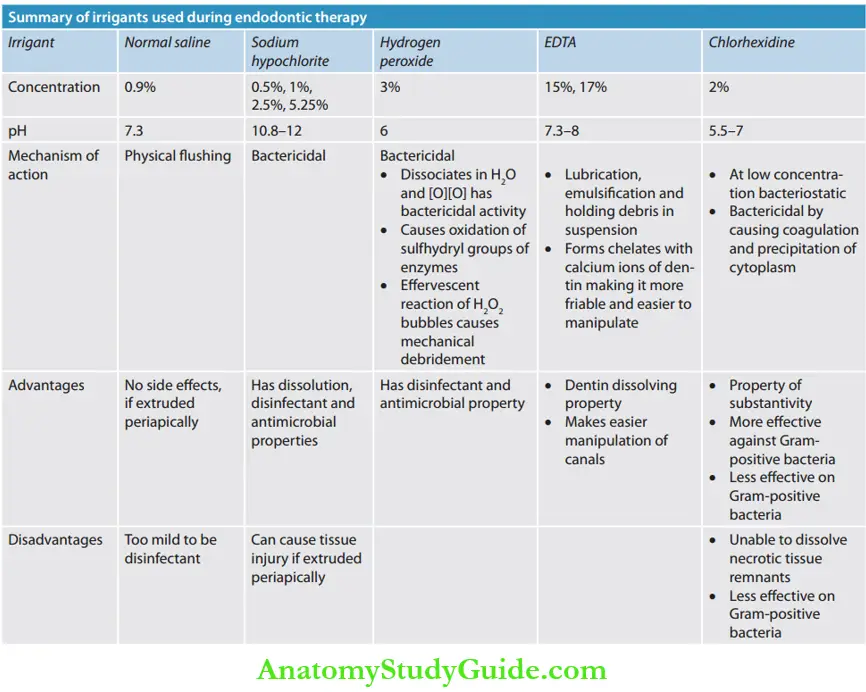
Normal Saline
Normal saline at 0.9% w/v is commonly used in endodontics for gross debridement and lubrication of root canals because it acts by fishing action. Since it is very mild in action, it can be used as an adjunct to chemical irritants. It can also be used as a final rinse for root canals to remove any chemical irritant left after root canal preparation.
Normal Saline Advantages:
It is biocompatible in nature. No adverse reaction even if extruded
periapical because the osmotic pressure of normal saline is the same as
that of the blood.
Normal Saline Disadvantages:
- Does not possess dissolution and disinfecting properties
- Too mild to thoroughly clean the canals
- Cannot clear microbial flra from inaccessible areas like accessory canal
- Does not possess antimicrobial activity
- Does not remove the smear layer
Sodium Hypochlorite
Sodium hypochlorite is a clear, pale, green-yellow liquid with a strong odor of chlorine. It was introduced during World War I by chemist Henry Drysdale Dakin for treating infected wounds. It is known as Dakin’s solution.
The original concentration suggested by Dakin was 0.5%. Besides being a wide spectrum, it is sporicidal, has tissue-dissolving properties. Due to these properties, Coolidge suggested the use of hypochlorite as an endodontic irrigant in 1919.
Availability:
- Unbuffered at pH 11 at conc. 0.5% to 5%
- Buffered with bicarbonate at pH 9.0 as 0.5% or 1% solution
Mechanism of Action of Sodium Hypochlorite:
- At body temperature, reactive chlorine in an aqueous solution exists in two forms—hypochlorous acid (HOCl) and hypochlorite (OCl) depending on the pH of the solution. On coming in contact with organic tissues:
- It forms glycerol and fatty acid salts (saponification reaction), resulting in the surface tension of the solution.
- It causes an amino acid neutralization reaction resulting in the formation of salt and water. pH decreases due to the release of hydroxyl ions.
- When hypochlorous acid comes in contact with organic tissue, it releases chlorine which combines with amino acids forming chloramines. This chlorination reaction between chlorine and amino acids causes interference in cell metabolism.
Together these three reactions that occur in the presence of organic tissues lead to tissue dissolution and an antibacterial effect.
Methods to Increase the Efficy of Sodium Hypochlorite:
Time:
The antimicrobial effectiveness of sodium hypochlorite is directly related to its contact time with the canal.
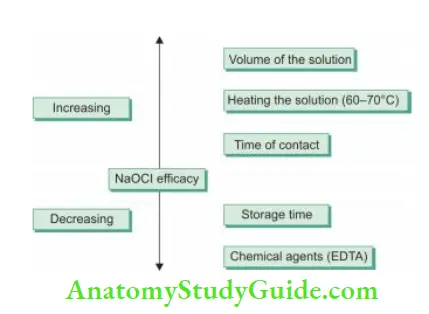
Heat:
An increase in temperature by 25°C enhances efficacy by a factor of 100. But one should be careful not to overheat the solution because this can cause the breakdown of sodium hypochlorite constituents and thus may damage the solution.
pH:
If NaOCl is diluted, its tissue dissolving property decreases. In an aqueous solution, hypochlorous acid dissociates into hypochlorite:
HOCl ↔ H+ + OCl–
HOCl is a stronger oxidant than hypochlorite ion, i.e. HOCl is responsible for strong chlorination, oxidizing action, and tissue dissolution. The dissociation of HOCl to OCl depends on pH. At pH 10, the OCl form exists and at pH of 4.5, the HOCl form dominates. So, the antibacterial properties of hypochlorite are more in acidic pH.
Ultrasonic Activation of Sodium Hypochlorite:
Ultrasonic activation of sodium hypochlorite has been shown to accelerate the chemical reactions, create a cavitational effect and thus achieve a superior cleansing action.
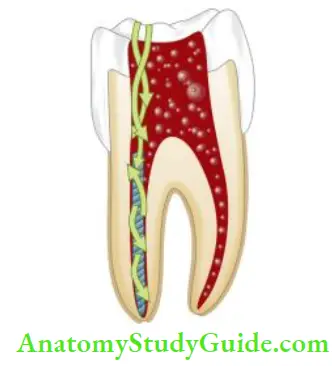
Precautions to be Taken while Using Sodium Hypochlorite Solution:
It is important to remember that though sodium hypochlo- rite is nontoxic during intracanal use but 5.25% NaOCl can cause serious damage to tissue if injected periodically. If sodium hypochlorite gets extruded into periapical tissues, it causes excruciating pain, periapical bleeding, and swelling.
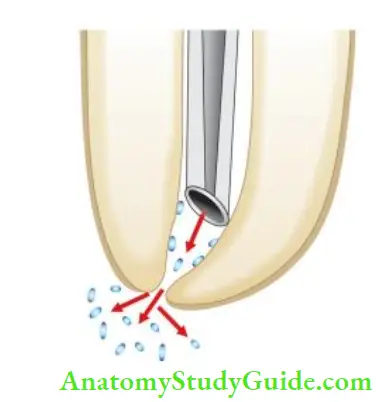
As the potential for the spread of infection is related to tissue destruction, medication like antibiotics, analgesics, and anti-histamine should be prescribed accordingly. In addition to these, reassurance to the patient is the prime consideration. Therefore, to avoid accidental extrusion of hypochlorite, care should be taken to do passive irrigation, especially in cases with large apical openings.
Precautions to be Taken while Using Sodium Hypochlorite Solution Advantages:
- Causes tissue dissolution
- Remove an organic portion of dentin for deeper penetration of medicament
- Removes biofilm
- Causes dissolution of pulp and necrotic tissue
- Shows antibacterial and bleaching action
- Causes lubrication of canals
- Economical
- Easily available
Precautions to be Taken while Using Sodium Hypochlorite Solution Disadvantages:
- Because of high surface tension, its ability to wet dentin is less
- Irritant to tissues, if extruded periodically, it can cause tissue
damage - If comes in contact, it causes inflammation of the gingiva because of its caustic nature
- It can bleach the clothes, if spillage occurs
- It has a bad odor and taste
- Vapors of sodium hypochlorite can irritate the eyes
- It can be corrosive to instruments
- It is unable to remove inorganic components of the smear layer
- Long time of contact with dentin has determined the effect on flexural strength of dentin
- Exudate and microbial biomass inactivate NaOCl. So, continuous irrigation and time are important when irrigation is done with NaOCl
Hydrogen Peroxide
It is a clear, odorless liquid and mainly a 3% solution of hydrogen peroxide is used as an irrigating agent.

Mechanism of Action:
- It is highly unstable and easily decomposed by heat and light. It rapidly dissociates into H2O + [O] (water and nas- cent oxygen). On coming in contact with tissue enzymes catalase and peroxidase, the liberated [O] produces bac- viricidal effect but this effect is transient and diminishes in the presence of organic debris
- It causes oxidation of the bacterial sulfhydryl group of enzymes and thus interferes with bacterial metabolism
- Rapid release of [O] on contact with organic tissue results in effervescence or bubbling action which is thought to aid in mechanical debridement by dislodging particles of necrotic tissue and dentinal debris and floating them to the surface
Hydrogen Peroxide Uses:
It is used as an irrigating solution either alone or alternatively with sodium hypochlorite. The advantage of using alternating solutions of 3% H2O2 and 5.2% NaOCl are
- The effervescent reaction by hydrogen peroxide bubbles pushes debris mechanically out of the root canal
- The solvent action of sodium hypochlorite on organic debris
- Disinfecting and bleaching action by both solutions
Hydrogen Peroxide Clinical Tips:
While using a combination of sodium hypochlorite and hydrogen peroxide, always use sodium hypochlorite in the last because hydrogen peroxide can react with pulp debris and blood to produce gas (nascent oxygen) which builds up pressure on closing the tooth, this can result in severe pain.
Urea
It is a white, odorless, crystalline powder. It was used in World War I as a therapeutic agent for infected wounds. Urea solution (40% by weight) is a mild solvent of necrotic tissue and pus and is a mild antiseptic too. In 1951, Blechman and Cohen suggested that 30% urea solution can be used as a root canal irrigant in patients with vital pulp as well as those with necrotic pulp.
Mechanism of Action:
- Denaturation of protein: Urea denatures the protein by destroying bonds of the secondary structure resulting in the loss of functional activity of the protein. This mode of action is responsible for its antiseptic property
- It has the property of chemically debriding the wound by softening the underlying substrate of firing
Urea Uses:
- It is an excellent vehicle for antimicrobials such as sulfonamides
- It has low toxicity
- It can be used in teeth with open apices or areas with resorptive defects
Urea Peroxide
It is a white crystalline powder with a slight odor. It is soluble in water, alcohol, and glycerine.
Mechanism of Action:
- It decomposes rapidly when exposed to heat, light, or moisture. It dissociates into urea and hydrogen peroxide
- Urea peroxide → Urea + H2O2
- Its mechanism of action combines the effects of urea and hydrogen peroxide.
- Anhydrous glycerol increases the stability of urea peroxide
Urea Peroxide Uses:
- A 10% solution of urea peroxide in an anhydrous glycerol base is available as a glycoside. The advantages of adding glycerol are
- It increases the stability of the solution, thus increasing shelf life
- It acts as a good lubricant, so facilitates the negotiation and instrumentation of thin, tortuous root canals
- Glyoxide can be used along with EDTA- Ethylenediaminetetraacetic acid to clean the walls of the canal
Urea Peroxide Disadvantages:
It dissociates more slowly than hydrogen peroxide (H2O2). So, its effervescence is prolonged but not pronounced. This can be overcome by alternating irrigation with sodium hypochlorite.
Chlorhexidine
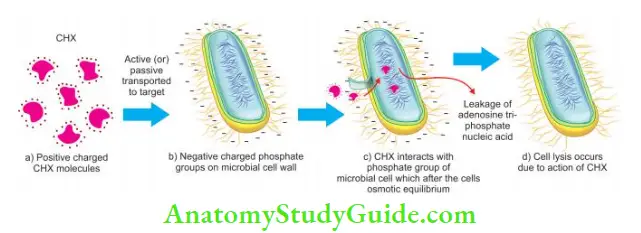
Chlorhexidine (CHX) is the most potent of tested bisbi- guanides. It has a strong base and is most stable in the form of its salts, that is, chlorhexidine gluconate. It shows optimal antimicrobial action between pH 5.5 and 7.0. For canal irrigation, it is used in 2% concentration.
Mechanisms of Action:
- Chlorhexidine is a broad-spectrum antimicrobial agent which is due to its cationic bisbiguanide molecular structure
- The cationic molecule is absorbed to negatively charged phosphate groups of the microbial cell walls. This alters the cell’s osmotic equilibrium and causes leakage of intracellular components
- At low concentrations, it acts as a bacteriostatic, whereas at higher concentrations, it causes coagulation and pre- precipitation of cytoplasm and therefore acts as bactericidal
- Chlorhexidine has the property of substantivity (residual effect). It can show residual antimicrobial activity for 72 h or even up to 7 days if used as an endodontic irrigant
Chlorhexidine Disadvantages:
- It is unable to dissolve necrotic tissue remnants
- It is less effective on Gram-negative than on Gram-positive bacteria
- Does not show the effect on biofilms
A combination of NaOCl and CHX is preferred to enhance their antimicrobial properties. However, the presence of NaOCl in the canals during irrigation with CHX produces an orange–brown precipitate known as parachloroaniline (PCA). This precipitate occludes the dentinal tubules and may compromise the seal of the obturated root canal.
Moreover, leaching of PCA from the insoluble precipitate has shown to be cytotoxic in rats and carcinogenic in humans. Formation of the precipitate can be prevented by minimizing the chance for two irrigants to come in contact with each other. Basrani et al. advocated fishing of remaining NaOCl with alcohol or EDTA, before using CHX.
Chelating Agents
A chelating agent is defined as a chemical which combines with a metal to form a chelate. Chelating agents were introduced in dentistry in 1957 to aid in the preparation of narrow and tortuous canals to soften the canal dentin, increase dentin permeability and remove the smear layer.
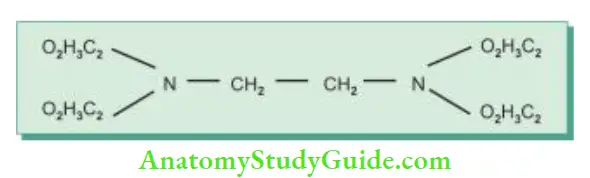
EDTA:
Ethylenediaminetetraacetic acid (EDTA) is the most commonly used chelating agent. It was introduced in dentistry Mechanism of action of chlorhexidine. by Nygaard-Ostby for cleaning and shaping of the canals. It contains four acetic acid groups attached to ethylene-amine. EDTA is relatively nontoxic and slightly irritating in weak solutions. The effect of EDTA on dentin depends on its concentration and duration of contact with dentin.
Chelating Agents Clinical Tips:
EDTA and citric acid are used for 2–3 min at the end of instrumentation to remove the smear layer so as to improve the antibacterial effect of locally used disinfecting agents in the deeper layer of dentin
EDTA or citric acid should never be mixed with sodium hypochlorite because EDTA and citric acid strongly interact with sodium hypochlorite. This immediately reduces the available chlorine in the solution and thus making it ineffective against bacteria
Functions of EDTA:
- Lubrication
- Emulsification
- Holding debris in suspension
- Smear layer removal
Mechanism of Action:
- It inhibits the growth of bacteria and ultimately destroys them by starvation because EDTA chelates with the metallic ions in a medium which are needed for the growth of microorganisms
- EDTA has self-limiting action. It forms a stable bond with calcium and dissolves dentin, but when all chelating ions are reacted, an equilibrium is reached which prevents further dissolution. According to Goldberg and Speilberg, the demineralization effect of EDTA is more at neutral pH than at alkaline pH.
- EDTA removes the smear layer within 1 minute of exposure and this effect doubles after 15 minutes of exposure. Further, an increase in time of contact does not increase the demineralization effect. This may be because of chelating material which starts to affect the dentin surface causing slower phosphorus release. This suggests refreshing the EDTA solution after 15 minutes.
- Calt and Serper found that increasing the contact time along with concentration from 10% to 17% and pH 7.5 to 9.0 has been shown to increase dentin demineralization.
Uses of EDTA:
- It has dentin-dissolving properties
- It helps in enlarging narrow canals
- Makes easier manipulation of instruments
- Reduces the time needed for debridement
Diffrent Preparations of EDTA:
- Liquid type:
- REDTA: In this, 17% EDTA is combined with cetrimide, that is, cetyltrimethylammonium bromide to reduce the surface tension.
- EDTAT: EDTA is combined with sodium lauryl ether sulfate (Tegretol) which reduces the surface tension.
- EDTAC: It is commercially available as a 15% solution with and pH of 7.3 under the name EDTAC because it contains catalog, a quaternary ammonium compound which is added due to its disinfecting properties. It reduces the contact angle of EDTA when placed on a dentin surface and thus enhances its cleaning efficacy.
- Largal Ultra: It contains 15% EDTA solution as a disodium salt, cetrimide, and sodium hydroxide to adjust pH value to 7.4.
- Paste type:
- Calcinase slide: It contains 15% sodium EDTA and 58%–64% water but no peroxides or preservatives. It has stable pH of 8–9. This gel is thixotropic in nature, so it is firm at room temperature and forms a creamy consistency when agitated. So it sticks to the instrument very well and spreads well in the root canal.
- RC prep: It consists of 10% urea peroxide, 15% EDTA and glycol in an ointment base. The presence of glycol makes it a lubricant and coats the instrument which facilitates its movement in the canal.
- Glyde fie: It consists of 10% urea peroxide, 15% EDTA in an aqueous solution. In reaction with NaOCl, oxygen is released from urea peroxide which causes effervescence. This facilitates the removal of pulpal remnants and debris.
- File-Eze: It contains 19% EDTA in an aqueous water-soluble solution.
Diffrent Preparations of EDTA Clinical Tips:
Collagen is a major constituent of vital pulp which can be packed into a glue-like mass which contributes to iatrogenic blocks. Without the use of a chelator, vital tissue tends to collapse and adheres to itself, but the use of a chelator does not allow this phenomenon to occur and accelerates the emulsification of tissue
Citric Acid:
- The use of 10% citric acid has been shown to remove the smear layer. It reacts with metals to form non-ionic chelate. It shows antimicrobial activity against facultative and obligative anaerobes
- Citric acid should not be used with sodium hypochlorite as it interacts with NaOCl and reduces the available chlorine making it ineffective against microorganisms
Polyacrylic Acid;
It is commercially available as Durelon and Fuji II liquid. Polyacrylic acid and 7% malic acid may be used to remove the smear layer.
Hydroxyethylidene Bisphosphonate:
- It is also known as etidronate or etidronic acid
- Hydroxyethylidene bisphosphonate (HEBP) is a nontoxic chelating agent and shows only short-term interference with sodium hypochlorite.
Maleic Acid:
It is a mild organic acid used as a conditioner in adhesive dentistry.
Salvizol:
- It belongs to surface-acting materials like the quaternary ammonium group. It shows antibacterial properties even in the presence of organic materials
- It is most effective against Gram-positive and Gram-negative microorganisms and fungi
Tetraclean:
It is a mixture of doxycycline hyclate [lower concentration than a mixture of a tetracycline isomer, an acid, and a detergent (MTAD)], an acid, and a detergent. It has been shown to remove microorganisms and smear layers from dentinal tubules with a fial 5-min rinse.
Chlorine Dioxide:
Chlorine dioxide is similar to hypochlorite and studies have shown that ClO 2 is equally efficient to NaOCl for dissolving organic tissue. Since, ClO2produces little or no trihalomethane- anes (carcinogen), it might prove as better dental irrigant than NaOCl.
Ultrasonic Irrigation
Ultrasonic irrigation has shown to clean the root canals or eliminate bacteria from the walls better than conventional methods. The use of ultrasonics causes a continuous flow of an irritant in the canal, thus preventing the accumulation of debris in the canal.
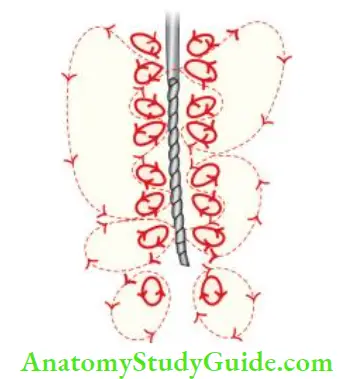
Mechanism of Action:
When a small file is placed in the canal and ultrasonic activation is given, ultrasonic energy passes through the irrigating solution and exerts its “acoustic streaming or scrubbing” effect on the canal wall. This mechanical energy warms the irrigant (sodium hypochlorite) and dislodges debris from the canal. The combination of activation and heating of irrigating solution is adjunct in cleaning the root canal.
Ultrasonic Irrigation Advantages:
- It cleans the root canal walls better than conventional ones
- It removes the smear layer efficiently
- It dislodges the debris from the canal better due to the acoustic effect
Ultrasonic Irrigation Disadvantages:
- Ultrasonic preparation of the canal is found to be unpredictable
- It can lead to excessive cutting of canal walls and may damage the finished preparation
Newer Irrigating Solutions
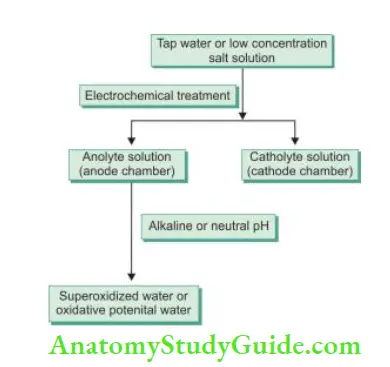
Electrochemically Activated Solution:
- It is produced from tap water and low-concentrated salt solutions
- The principle of electrochemically activated (ECA) is transferring liquid into the metastable state via electrochemical unipolar action using a reactor
- Electrochemical treatment in anode and cathode chambers results in the synthesis of two types of solutions, that is, anolyte (produced in anode chamber) and catholyte (produced in cathode chamber)
- Anolyte solution has also been termed as super oxidized water or oxidative potential water. The pH of an anolyte can be acidic (anolyte), neutral (anolyte neutral), or alkaline (anolyte neutral cathodic). Earlier, acidic anolyte was used but now neutral and alkaline solutions are preferred
Advantages of electrochemically activated solution:
- Nontoxic to biological tissues
- Less or no allergic reaction
- Effctive with a wide range of microbial spectra
- Combined use of NaOCl and ECA solution has been shown to remove the smear layer
Ozonated Water Irrigation:
- Ozone is an unstable gas which can oxidize any biological unit. Ozonated water is shown to be a powerful antimicrobial agent against bacteria, fungi, protozoa, and viruses
Advantages of ozonated water:
- Its potency
- Ease of handling
- Lack of mutagenicity
- Rapid microbial effects
Ruddle’s Solution:
It is of 17% EDTA, 5% NaOCl and hypaque.
Mechanism of Action:
- Hypaque is an aqueous radiopaque solution of iodide salts, namely, diatrizoate and sodium iodine
- The use of EDTA lowers the surface tension and allows better penetration of sodium hypochlorite
- The solvent action of sodium hypochlorite clears the contents of the root canal system and thus enables hypaque component to flow into every nook and corner of the canal system such as fracture, perforation, missed canals, and defective restoration
Ruddle’s Solution Uses:
- Useful for visualization of root canal anatomy, missed canal, perforation, etc.
- Helps in the diagnosis of internal resorption, its size, and site
- Helps in the visualization of blockage, perforation, ledge, and canal transportation
- Helps in the management of iatrogenic errors
Photoactivated Disinfection:
Photoactivated disinfection (PAD) is based on the concept that nontoxic photosensitizers can be localized in certain tissues and activated by light of the suitable wavelength to produce oxygen and free radicals which are cytotoxic to cells of the target tissue. Methylene blue, toluidine blue, and chlorine p6 are commonly used photosensitizers which release oxygen when exposed to the low-power laser.
Advantages of PAD:
- Most effctive antimicrobial agent
- Effectively kills Gram-negative, Gram-positive, aerobic and anaerobic bacteria
- Overcomes the problems of antibiotic resistance
- Kills bacteria present in complex biofilm such as sub-gingival plaque which is typically resistant to the action of antimicrobial agents
- Does not pose any thermal risk due to the low power of the PAD laser
- Does not cause any sensitization
- Nontoxic
A Mixture of a Tetracycline Isomer, an Acid, and a Detergent (MTAD):
MTAD was introduced in 2000 as a final rinse for disinfection of root canal system. Torabinejad et al. have shown that MTAD is able to safely remove the smear layer and is effctive against Enterococcus faecalis.
Composition:
- Tetracycline:
- It is a bacteriostatic broad-spectrum antibiotic
- It has low pH and acts as a calcium chelator
- It removes the smear layer
- It has the property of substantivity
- It promotes healing
- Citric acid: It is bactericidal in nature and removes the smear layer
- Detergent (Tween 80): It decreases surface tension
Advantages of MTAD:
- It is an effctive solution for the removal of the smear layer
- It kills E. faecalis which has been shown to be resistant to many intracanal medicaments and irrigants
- It is biocompatible
- MTAD has similar solubilizing effects on pulp and dentin to those of EDTA
- The high binding affinity of doxycycline present in MTAD for dentin allows a prolonged antibacterial effect (it is the main difference between MTAD and EDTA)
Q-MIX:
Q-Mix 2 in 1 is a colorless and odorless solution which consists of 17% EDTA and 2% chlorhexidine which can kill 99.99% of the bacteria. To be used as a final rinse, continuous irrigation of root canal is done for 60–90 s.
Functions:
Kills 99.99% of planktonic bacteria
Penetrates biofilm
Advantages of Q-MIX:
- Less demineralization of dentin as compared to EDTA
- It does not cause erosion of dentin like NaOCl when NaOCl is used as a final rinse after EDTA
Herbal Irrigants
Herbal irrigants are becoming popular now due to their biocompatibility, antimicrobial activity, and antioxidative and anti-inflammatory nature. The following are commonly used herbal irrigants:
Triphala and Green Tea Polyphenols:
Triphala’s fruit is rich in citric acid. It has a chelating property which helps in removing the smear layer. Green tea polyphenols possess antioxidant, anti-cariogenic, anti-inflammatory, and antimicrobial properties. J. Prabhakar et al. showed that Triphala and Green tea polyphenols have significant antimicrobial activity against E. faecalis biofilm.
Turmeric:
It possesses anti-inflammatory, antioxidant, antimicrobial, and anticancer activity. Studies have shown its antibacterial activity against E. faecalis and thus can be used as for root canal irrigation.
German Chamomile and Tea Tree Oil:
The active component of tea tree oil is terpinene-4-ol which possesses anti-inflammatory, analgesic, and antimicrobial properties. It helps in removing the smear layer and has activity against E. faecalis.
Allium sativum (Garlic):
Its active component is allicin which destroys the bacterial cell wall and thus can be used as root canal irrigant.
Azadirachta indica (Neem):
Neem possesses antifungal, antibacterial, antioxidant, and anticarcinogenic activity. Naiyak Arathi et al. in their study showed that ethanolic extract of neem has significant activity against E. faecalis.
Propolis:
Propolis is a resinous substance which honey bees collect from poplars and conifers. It shows antioxidant, anti-inflammatory and antibacterial activities against Streptococcus sobrinus and Streptococcus mutans. Studies have shown its antimicrobial activity comparable to that of sodium hypochlorite.
Myristica fragrans (Nutmeg):
Its main constituent myristic acid has antibacterial properties.
Spilanthes Calva DC:
Moulshree Dube et al. showed that the antibacterial efficacy of the methanolic extract of Spilathes calva DC is comparable to sodium hypochlorite.
Acacia nilotica (Babool):
Acacia nilotica have antimicrobial, antioxidant, and antibiotic properties. Research have shown that a 50% concentration of acacia shows the highest activity against E. faecalis.
Aloe vera:
Aloe vera has antibacterial and antifungal activity. It has been found to be effctive against E. faecalis and resistant microorganisms of root canals.
Method Of Irrigation
- The solution should be introduced slowly and passively into the canal
- The needle should never be wedged into the canal and should allow an adequate backflow
- The blunted needles of 26 gauge or 27 gauge is preferred
- In the case of small canals, deposit the solution in a pulp chamber. The fie carries the solution into the canal. The capillary action of a narrow canal will stain the solution.
- To remove the excess fluid, either the aspirating syringe or a 2 × 2 inches folded gauze pad is placed near the chamber. To further dry the canal, remove the residual solution with paper point
- Regardless of the delivery system, irrigants must never be forcibly inserted into apical tissues
- For effctive cleaning, the needle delivering the solution should be in close proximity to the debris to be removed
- In the case of large canals, tip of the needle should be introduced until resistance is felt, then withdraw the needle 2–3 mm away from that point and irrigate the canal passively. For removal of the solution, a sterile gauge pack or paper points should be used
- In order to clean effectively in both anterior and posterior teeth canals, a blunt bend of 30° in the center of the needle can be given to reach the optimum length to the canal
- The volume of irrigant is more important than concentration or type of irrigant
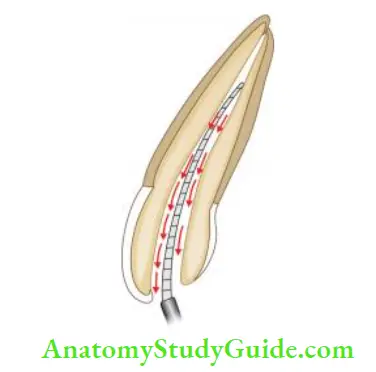
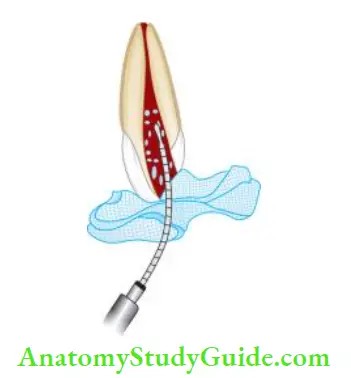
Various delivery systems for irrigation:
- Stropko irrigator
- 27-gauge needle with a notched tip
- Needle with bevel
- Monojet endodontic needle
- 23-gauge
- 27-gauge
- ProRinse—25-, 28-, 30-gauge probes
- Ultrasonic handpiece
Ideal properties of irrigating needle:
- An irrigating needle should
- Blunt
- Allow backflow
- Flexible
- Longer in length
- Easily available
- Cost-effctive
Different Needle Designs:
- Stropko Irrigator:
In this system, a combination of delivery and recovery of irrigant is present in one probe. It is specially used when a surgical operating microscope is used for procedures.
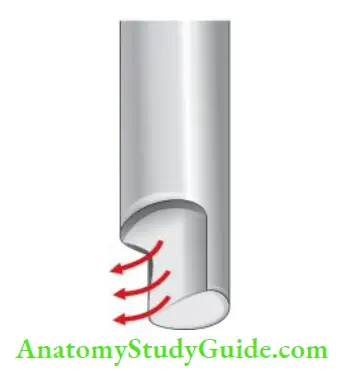
27-Gauge Needle with Notched Tip:
This needle is preferred as its notched tip allows backflow of the solution and does not create pressure in the periapical area. So, it ensures optimum cleaning without damage to the periapical area.
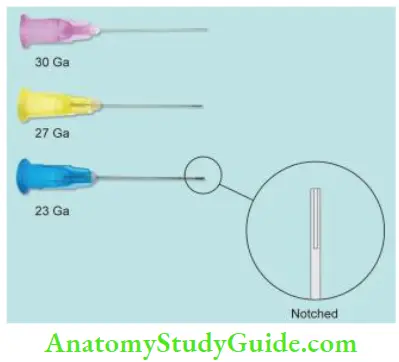
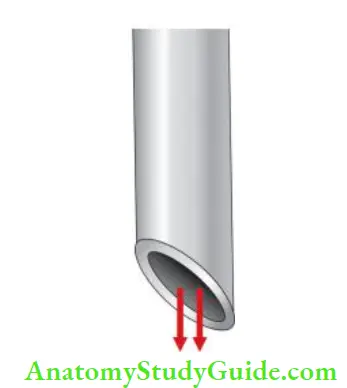
Needle with Bevel:
Needle with bevel, if gets lodged into the canal, there is a risk of forcing irrigant past the apex.
Monojet Endodontic Needle:
This is an efficient irrigant long blunt needle that can be inserted to the full length of the canal to ensure optimum cleaning.
ProRinse probe:
The design of this needle produces an upward flushing motion for complete canal irrigation. Its side port dispersal pre- vents solution and debris from being expressed through the apex and closed, rounded end reduces the risk of apical damage
Micro brushes and Ultrasonic:
In this, bristles are attached to braided wires or flexible plastic cores. An optimal-sized micro brush can be attached to a rotary or ultrasonic handpiece. These micro brushes have tapers like nonstandardized gutta-percha cones. These are used in conjunction with sodium hypochlorite and EDTA to produce clean canals.
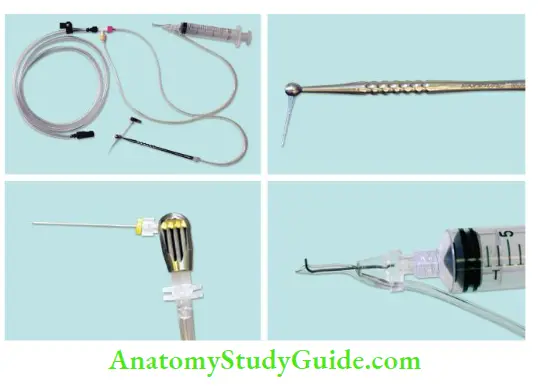
Endovac (Apical Negative Pressure Irrigation System):
The EndoVac apical negative pressure irrigation system draws fluid apically by way of evacuation. Instead of applying positive pressure, it uses suction to pull the irrigant down the canal. This system is comprised of the following parts:
- Master delivery tip, which allows a constant flow of irrigant without overflow
- Microcannula, which removes coarse debris left in the canal from instrumentation
- Microcannula, which removes microscopic debris at the apical 1 mm via 28 gauze needle with 12 laser-drilled microscopic evacuation holes
Precautions:
- Confirm the integrity of the rubber dam seal
- Protect patient’s eyes and clothing from sodium hypochlorite spill
- Never place the MDTs delivery tip closer than 5 mm from the coronal opening of the canal
- For optimal use of the EndoVac system, the canal should be instrumented to a minimum of 35 No. at a 4% taper or 45 No, if 2% taper
- Make sure no air bubbles are trapped in the prefilled syringes, as this will cause uncontrolled irrigant extrusion after releasing the plunger pressure
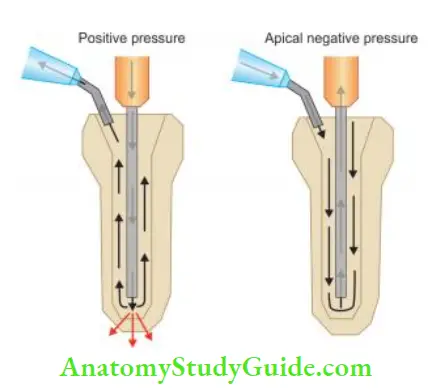
Positive Pressure versus Apical Negative Pressure:
Irrigation involves the placement of an irrigating solution into the canal system and its evacuation from the tooth. It is done by placing an end-port or side-port needle into the canal and expressing solution out of the needle to be suc- turned coronally. This creates a positive pressure system with force created at the end of the needle, which may lead to the solution being forced into the periapical tissues.
In an apical negative pressure irrigation system, the irrigation solution is expressed coronally, and suction at the tip of the irrigation needle at the apex creates a current flow down the canal toward the apex and is drawn up the needle. But true apical negative pressure only occurs when the needle is used to aspirate irrigants from the apical termination of the root canal.
The apical suction pulls irrigating solution down the canal walls toward the apex, creating a rapid, turbulent current force toward the terminus of the needle.
Intracanal Medicaments
Originally, endodontics was mainly a therapeutic procedure in which drugs were used to destroy microorganisms, fi or mummify vital tissue and affect the sealing of the root canal space.
The drugs commonly used were caustics such as phenol and its derivatives which were shown to produce adverse effects on the periapical tissues. Gradually, the reliance on drugs has been replaced by an emphasis on thorough canal debridement. But drugs are still being used as intratreat- ment dressings, although an ever-increasing number of endodontists use them only for symptomatic cases.
Functions of Intracanal Medicaments:
- Destroy the remaining bacteria and limit the growth of new arrivals
- In cases of apical periodontitis, for example, in cases of inflammation caused due to over instrumentation
Indications of Using Intracanal Medicaments:
- Remove the remaining microorganisms from the pulp space
- Dry the weeping canals
- Act as a barrier against leakage from an interappointment dressing
- Neutralize the tissue debris
Desirable Properties of an Intracanal Medicaments
It should
- Be effctive germicide and fungicide
- Be nonirritating to pulpal tissue
- Remain stable in the solution
- Have prolonged antimicrobial action
- Remain active in the presence of blood, pus, etc.
- Have low surface tension
- Not interfere with repair of periapical tissue
- Non-staining to tooth
- Be capable of inactivation in the cultural media
- Not induce an immune response
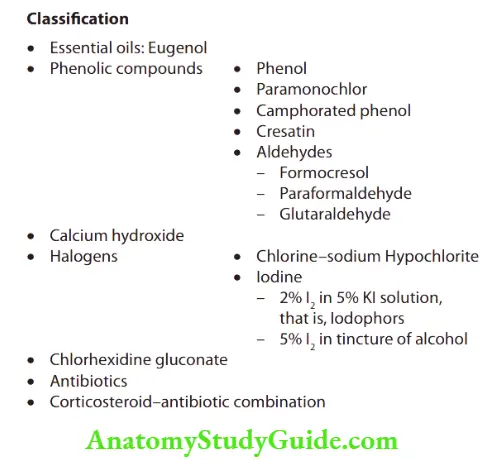
Classifiation Of Intracanal Medicaments
Characteristics Of Intracanal Medicaments
Essential Oils:
Eugenol
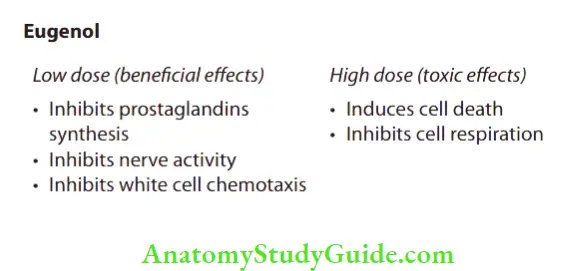
It has been used in endodontics for many years. It is a constituent of most root canal sealers and is used as a part of many temporary sealing agents. This substance is the chemical essence of the oil of clove and is related to phenol. The effects of eugenol are dependent on tissue concentrations of the eugenol and can be divided into low doses (beneficial effects) and high doses (toxic effects).
Uses of eugenol:
Used as an intracanal medicament
Used as root canal sealers
Part of temporary sealing agents
Phenolic Compounds:
Phenol:
It was used for many years for its disinfectant and caustic action. However, it has strong inflammatory potential, so, at present, it is rarely used as an intracanal medicament. Liquefied phenol (carbolic acid) consists of nine parts of phenol and one part of water.
Phenolic Compounds Uses:
- It is used for disinfection before periapical surgery
- It is also used for cauterizing tissue tags that resist removal with broaches or fees
Parachlorophenol:
Parachlorophenol (PCP) has been a very popular component of dressing as phenol is no longer used in endodontics because of its high toxicity-to-efficacy ratio.
Parachlorophenol Composition:
- It is a substitution product of phenol in which chlorine replaces one of the hydrogen atoms (C6H4OHCl)
- On trituration with gum camphor, these products combine to form an oily liquid
Parachlorophenol Concentration: 1% aqueous solution is preferred.
Parachlorophenol Uses: Used as a dressing of choice for an infected tooth
Camphorated Monoparachlorophenol (CMCP)
It is probably the most commonly used medicament in endodontics, presently, even though its use has decreased considerably in the past few years.
Camphorated Monoparachlorophenol Composition:
Two parts of PCP
+
Thess parts gum camphor
↓
Camphorated monochlorophenol (CMCP)
Camphor is added to PCP because it
- Has diluent action
- Prolongs the antimicrobial effect
- Reduces the irritating effect of PCP
- Serves as a vehicle for the solution
Camphorated Monoparachlorophenol Uses: Used as a dressing of choice for infected teeth.
Cresatin:
Schilder and Amsterdam showed that Cresatin possesses the same desirable qualities and actions as that of CMCP, but is less irritating to periapical tissues
Cresatin Composition: It is a clear, stable, oily liquid of low volatile
nature is known as meta cresyl acetate.
Aldehydes:
- Formaldehyde, paraformaldehyde, and glutaraldehyde are commonly used intracanal medicaments in root canal therapy
- These are water-soluble protein denaturing agents and are considered among the most potent disinfectants
- They are mainly applied as disinfectants for surfaces and medical equipment which cannot be sterilized, but they are quite toxic and allergic and some even may be carcinogenic
Formocresol:
Formocresol contains formaldehyde as its main ingredient and is still widely used medicament for pulpotomy procedures in primary teeth but its toxic and mutagenic properties are of concern

Composition of formocresol:
- Formaldehyde—19%
- Cresol—35%
- Water and glycerine—46%
Formocresol Uses: Used as dressing for pulpotomy to fi the retained pulpal tissue.
Paraformaldehyde:
- It is a polymeric form of formaldehyde and is commonly found as a component of some root canal obturating material like beclomethasone
- It slowly decomposes to give out formocresol, its monomer
- Its properties are similar to formaldehyde that is toxic, allergenic, and genotoxic in nature
Paraformaldehyde Clinical Tips:
All phenolic and similar compounds are highly volatile with low surface tension. If they are placed on a cotton pellet in the pulp chamber, vapors will penetrate the entire canal preparation. Therefore, a paper point is not needed for their application. Only a small quantity of medication is needed for effctiveness, otherwise, chances of periapical irritation are increased.
Calcium Hydroxide:
The use of calcium hydroxide in endodontics was introduced by Hermann in 1920. It acts as a strong base in contact with aqueous solution and dissociates into calcium and hydroxyl ions.
Effects of Calcium Hydroxide:
- Physical
- Acts as a physical barrier for the ingress of bacteria
- Destroys the remaining bacteria by limiting space for multiplication and holding substrate for growth
- Chemical
- It shows antiseptic action because of its high pH and leaching action on necrotic pulp tissues. It also increases the pH of circumpolar dentin when placed into the root canal
- Suppresses enzymatic activity and disrupts the cell membrane
- Inhibits DNA replication by splitting it
- It hydrolyses the lipid part of bacterial lipopolysaccharide (LPS) and thus inactivates the activity of LPS. This is a desirable effect because dead cell wall material remains after the killing of bacteria which may cause infection
- Calcium hydroxide is available in
- Paste form: Single paste or in combination with iodoform
- Powder form: Powder form is mixed with saline and anesthetic solution. For placement in root canals, it is coated with the help of paper points, spreaders, or lentils spirals.
Indications of calcium hydroxide
- In weeping canals
- In the treatment of Phoenix abscess
- In resorption cases
- For specification
- During pulpotomy
- For nonsurgical treatment of periapical lesions
- In cases of direct and indirect pulp capping
- As a sealer for obturation
- To decrease postoperative pain after over instrumentation, it is used in combination with Ledermix (1:1)
Advantages of Ca(OH)2
- Inhibits root resorption
- Stimulates periapical healing
- Encourage mineralization
Disadvantages of Ca(OH)2 as an intracanal medicament
- Difficult to remove from canals
- Decreases setting time of zinc oxide eugenol-based cements
- It has little or no effect on the severity of post obturation pain
Use of Calcium Hydroxide in Weeping Canal Cases
Sometimes, a tooth undergoing root canal treatment shows constant clear or reddish exudation associated with periapical radiolucency. A tooth can be asymptomatic or tender on percussion. When opened in next appointment, exudates stop but it again reappears in the next appointment. This is known as the “weeping canal.”
In these cases, a tooth with exudates is not ready for filing, since culture reports normally show negative bacterial growth, so antibiotics are of no help in such cases. For such teeth, dry the canals with sterile absorbent paper points and place calcium hydroxide in the canal. By the next appointment, one finds a dry canal, ready for obturation.
It happens because the pH of periapical tissues is acidic in a weeping stage which gets converted into basic pH by calcium hydroxide. Some say that the caustic effect of calcium hydroxide burns the residual chronic inflamed tissue and also calcium hydroxide builds up the bone in the lesion due to its calcifying action.
Halogens:
Halogens include chlorine and iodine which are used in various formulations in endodontics. They are potent oxidizing agents with rapid bactericidal effects.
Chlorine:
Sodium hypochlorite: The disinfectant action of halogens is inversely proportional to their atomic weights. So, when compared to iodine, chlorine shows better disinfectant action. But chlorine disinfectants are not stable compounds because they interact rapidly with organic matter.
Mentz found sodium hypochlorite as an effctive intracanal medicament as well as an irrigant. As the activity of sodium hypochlorite is intense but of short duration, the compound should be changed in the root canal every other day.
Iodides:
Iodine is highly reactive in nature. It combines with proteins in a loosely bound manner so that its penetration is not impeded. It destroys microorganisms by forming salts that are unfavorable to the life of the organism. Iodine is used as iodine potassium iodide and in iodophors, which are organic iodine-containing compounds that release iodine over time.
It is also a very potent antibacterial agent of low toxicity but may stain clothing if spilled. It is used as an irrigating solution and short-term dressing in a 2% solution of iodine in 4% aqueous potassium iodide and as a constituent of gutta-percha points for filing.
2% Chlorhexidine Gluconate:
The antibacterial activity of chlorhexidine gluconate is comparable to sodium hypochlorite. Substantivity, broad-spectrum activity, and low toxicity of CHX make it suitable for irrigation. Attempts are being made to utilize its disinfecting properties in gutta-percha points.
PBSC Paste:
As mentioned by Grossman, PBSC has enjoyed wide use among dentists. The constituents of PBSC paste are as follows:
- Penicillin—effctive against Gram-positive microorganisms
- Bacitracin—effctive against penicillin-resistant microorganisms
- Streptomycin—effctive against the Gram-negative microorganisms
- Caprylate (sodium salt)—effctive against fungi
Nystatin replaces sodium caprylate as the antifungal agent and is available in the form of PBSN. Both are available in a paste form that may be injected into root canals or impregnated on paper points. Because there is no volatility, the drug must be placed in the canal to have effect in that area.
PBSC may interfere with subsequent culturing procedures; therefore, penicillinase may be added to culture media to inactivate penicillin. Reports of allergic reactions to the drug have been presented, if the patient reports a history of allergy to any of the constituents, the drug should not be used.
With the decline in popularity of intracanal drugs in general and because of the potential for sensitivity due to topical use of antibiotics, PBSN largely has fallen into disuse.
Sulfonamides:
Sulfanilamide and sulfathiazole are used as medicaments by mixing with sterile distilled water or by placing a moistened paper point into a fluffy jar containing the powder. Yellowish tooth discoloration has been reported after use. Sulfonamides are usually recommended while giving closed dressing in a tooth which had been left open after an acute periapical abscess.
N2 by Sargent:
It is a compound consisting of paraformaldehyde as the main ingredient. It contains eugenol, phenyl mercuric borate, and perfumes. Antibacterial effect of N2 is short-lived and dissipated in 7–10 days.
Grossman Paste
Composition
- Potassium penicillin G 1,000,000 units
- Bacitracin 100,00
- Streptomycin sulfate 1.0 g
- Sodium caprylate 1.0 g
- Silicon flid 3 mL
- Nystatin 10,000 units
Chloramines-T:
It is a chlorine compound with good antimicrobial. It is used in the concentration of 5%. It remains stable for a long period of time and is used to disinfect gutta-percha points. It can be used in patients allergic to iodine.
Quaternary Ammonium Compounds:
These are positively charged compounds which attract negatively charged microorganisms; they have low surface tension, for example, aminoacridine. Aminoacridine is a mild antiseptic which is more effc- tive than creation but less effctive than CMCP. It is used more as an irritant than intracranial medicament.
Corticosteroid–Antibiotic Combinations:
- Medications that combine antibiotic and corticosteroid elements are highly effctive in cases of over instrumentation
- They must be placed into the inflamed periapical tissue by a paper point or reamer
- Tetra-Cortril, Cortisporin, Mycolog, and other combinations are available for their use in endodontics
- Ledermix is one of the best known antibiotic-corticosteroid combinations.
- Schroeder and Triadan developed Ledermix in 1960. It contains an antibiotic demeclocycline—HCl (3.2%) and a corticosteroid, triamcinolone acetonide (1%), in a polyethylene glycol base.
- Corticosteroid constituent reduces the periapical inflmmation and gives almost instant relief of pain to the patient who complains of extreme tenderness to percussion after canal instrumentation
- Antibiotic constituents present in the corticosteroid–antibiotic combination prevent the overgrowth of micro-organisms when the inflammation subsides

Placement Of Intracanal Medicament
- Copiously irrigate the canal to remove debris present if any
- Place the master apical file in the canal
- Dry the canal using absorbent paper points
- Place the intracanal medicament on a sterile cotton pellet and place it in the pulp chamber
- Over this, another sterile cotton pellet is placed, which is finally sealed with a temporary restorative material
Limitations of Intracanal Medicaments:
- For an intracanal, medicament to be effctive, it should remain active during the time of interappointment, which does not happen not in every case
- Clinical effctiveness of sustained release delivery systems is unknown
- The therapeutic action of medicament depends upon its direct contact with tissues, but it can be prevented due to the presence of organic tissue/matter
Irrigation And Intracanal Medicaments Conclusion
The success of endodontic treatment depends on the eradication of microbes from the root-canal system and prevent- tion of reinfection. Instrumentation and irrigation are the most important parts of successful endodontic treatment. Irrigant performs many functions, the most important of which are to dissolve tissue and to have an antimicrobial effect.
Commonly used during cleaning and shaping include sodium hypochlorite, chlorhexidine, EDTA, MTAD, etc. None of these irrigants has all of the characteristics of an ideal irrigant. Many chemicals used for irrigation have been chemically modified and several mechanical devices have been developed to improve the penetration and effctive- ness of irrigation.
Intracanal medicaments have been used to disinfect root canals between appointments and reduce interappointment pain. The major intracanal medications currently used in endodontics include calcium hydroxide, though the search for an ideal material and/or technique to completely clean infected root canals continues.
Leave a Reply