Autoimmunity, Immunodeficiency, Transplantation, and Immunoprophylaxis
Autoimmunity
Table of Contents
Autoimmunity is a condition in which the body’s own immunologically competent T-cells or antibodies act against its self-antigens resulting in structural or functional damage.
Immunological Tolerance
Immunological tolerance is a state in which an individual is incapable of developing an immune response against his own tissue antigens.
It is mediated by two broad mechanisms—central tolerance and peripheral tolerance.
Central Tolerance
This refers to the deletion of self-reactive T and B lymphocytes during their maturation in central lymphoid organs.
Read And Learn More: Micro Biology And Immunology Notes
Peripheral Tolerance
This refers to several back-up mechanisms that occur in the peripheral tissues to counteract the self-reactive T-cells that escape central tolerance. It is provided by several mechanisms.
- Ignorance: The self-reactive T-cells might never encounter the self-antigen which they recognize and therefore remain in a state of ignorance.
- Anergy: By blocking co-stimulatory signal by binding of B7 molecules on APC to CTLA-4 molecules on T-cells
- Phenotypic skewing: Self-reactive T-cells stimulated by self-antigens secrete nonpathogenic cytokines
- Apoptosis of Self-reactive T-cells by activation-induced cell death (AICD)
- Regulatory T-cells (Treg cells) can downregulate the self-reactive T-cells
- Dendritic cells (DCs): When certain dendritic cells, such as immature DCs and tolerogenic
- DCs capture the self-antigen for processing, they downregulate the expression of molecules of co-stimulatory ligands, such as CD40 and B7 molecules or act indirectly by induction of regulatory T-cells.
- Sequestration of self-antigen in immunologically privileged sites, e.g. corneal proteins, testicular and brain antigens.
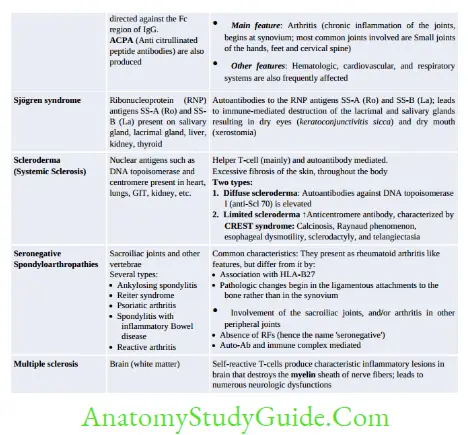
Mechanisms of Autoimmunity
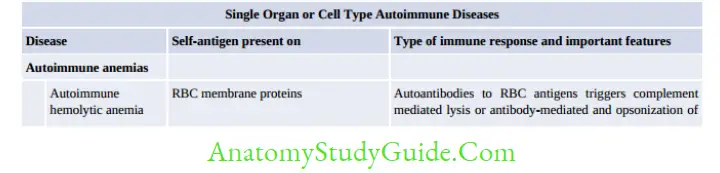
- Breakdown of CTLA-4 mediated T-Cell Anergy: Seen in multiple sclerosis, rheumatoid arthritis and psoriasis
- Failure of AICD (activation-induced cell death): Seen in SLE
- Loss of Treg cells
- Providing T-cell help to stimulate self-reacting B-cells
- Release of Sequestered Antigens (spermatozoa and ocular antigens) due to injury to organs
- Molecular Mimicry, e.g. in post-streptococcal acute rheumatic fever and glomerulonephritis
- Polyclonal Lymphocyte Activation: Mediated by
- Superantigens, EBV and HIV
- Exposure of cryptic self-epitopes
- Epitope spreading
- Bystander activation.


Immunodeficiency Disorders
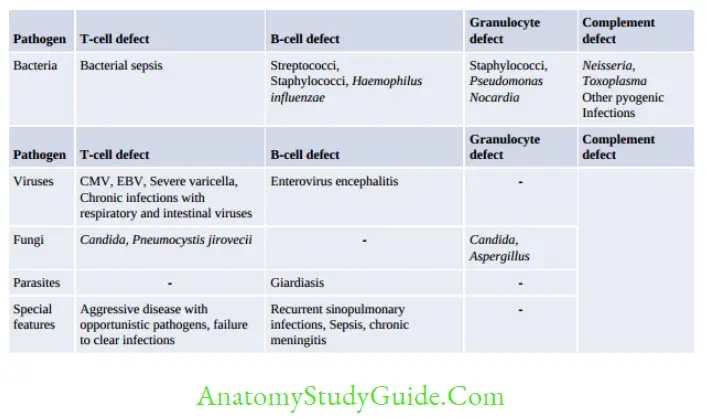
Immunodeficiency is a state where the defense mechanisms of the body are impaired, leading to enhanced susceptibility to microbial infections as well as to certain forms of cancer.
It is broadly classified as primary or secondary.
Primary immunodeficiency diseases result from inherited defects affecting immune system development.
- Secondary immunodeficiency diseases are secondary to some other disease process that interferes with the proper functioning of the immune system (e.g. infection, malnutrition, aging, immunosuppression, autoimmunity, or chemotherapy). They are more common than primary immunodeficiency diseases.








Transplant Immunology
Based on the genetic relationship between the donor and the recipient:
- Autograft is self-tissue transferred from one part of the body site to another in the same individual, e.g. skin grafts
- Isograft or syngeneic graft is tissue transferred between genetically identical individuals
- Allograft is tissue transferred between genetically non-identical members of the same species (e.g. most transplants)
- Xenograft is tissue transferred between different species (e.g. the graft of a baboon heart into a man)
Second set rejection of the graft is always faster than the first set rejection, i.e. if, in a recipient that has rejected a graft by the first set response, another graft from the same donor is transplanted, it will be rejected in an accelerated fashion.
Mechanism of Graft Rejection
The process of graft rejection can be divided into two stages:
- Sensitization phase: Involves alloantigen (mainly graft MHC molecules) presentation to recipient’s T-cells either by direct or indirect pathways:
- Direct pathway: The MHC molecules on graft’s APCs are directly presented to the recipient’s helper T-cells.
This pathway is responsible for most of the acute graft rejections mediated by cytotoxic T-cells. - Indirect pathway: This is similar to that for recognition of any foreign antigen by the host APCs.
The graft alloantigens are processed and presented by recipient APCs to recipient’s helper T-cells.
This pathway is responsible for most of the chronic rejection mediated by helper T-cells via specialized form of chronic DTH reaction.
- Direct pathway: The MHC molecules on graft’s APCs are directly presented to the recipient’s helper T-cells.
- Effector phase: This involves a variety of effector mechanisms leading to immune destruction of the graft such as:
- Cell-mediated reactions involving delayed-type hypersensitivity T-cells and cytotoxic Tcells.
- Antibody-mediated mechanisms: Complement mediated lysis and ADCC.
Laboratory Tests to Determine Histocompatibility
Prior to transplantation, various laboratory tests should be carried out to assess the histocompatibility:
- ABO blood group compatibility testing by blood grouping and cross-matching.
- Immunosuppressive therapy
- HLA typing: In this test, donor’s antigens expressed on the surface of leukocytes or their gene to that of recipient are matched. The HLA compatibility is determined by:
- Phenotypic method: Serology (Microcytotoxicity) and Tissue typing (Mixed lymphocyte reaction)
- Genotypic methods:
- PCR detecting HLA genes
- PCR-RFLP (restriction fragment length polymorphism)
- PCR-SSOP (PCR sequence-specific oligonucleotide probing)
- PCR-SSP (PCR-sequence-specific primer)
- PCR-DNA sequencing
- Conformational analysis.
(PCR-SSOP, PCR-SSP, and PCR-DNA sequencing are the most reliable methods currently in use; have shown high-resolution matching).
Graft-Versus-Host Reaction
GVH reaction is a condition, where graft mounts an immune response against the host (i.e.recipient) and rejects the host, in contrary to the usual situation where the recipient mounts an immune response against the graft antigens.
GVH reaction occurs in two forms. Acute or fulminant (occurs < 100 days) and chronic GVH disease (occurs after 100 days)
- The acute GVH disease is characterized by selective damage to the liver (hepatomegaly), skin (rash), mucosa, and the intestine (diarrhea) mediated graft’s immunocompetent T-cell.
- Chronic GVH disease also attacks the above organs, but in addition, it causes damage to the connective tissues and exocrine glands.
- Experimentally, GVH reaction can be produced in mice, called Runt disease.
- Treatment: Glucocorticoids.
- Tumor Antigens
Two types of tumor antigens have been identified on tumor cells:
- Tumor-specific transplantation antigen (TSTA): They are present only on tumor cells and are absent in normal cells.
TSTA are induced on tumor cells either by chemical or by physical carcinogens, and also by viral carcinogens.
In chemically/physically induced tumors, the TSTA is tumor-specific. Different tumors possess different TSTA, even though induced by the same carcinogen.- In contrast, the TSTA of virus-induced tumors is virus-specific; all tumors produced by one virus would possess the same antigen.
- Tumor-associated antigens (TATAs): In addition to tumor cells, they may also be expressed by normal cells but at a very low level, e.g.
Oncofetal antigens, Carcinoembryonic antigen (CEA), Carbohydrate antigens (CA 125, CA 19-9), prostate-specific antigen and β2 macroglobulin.
Immunoprophylaxis


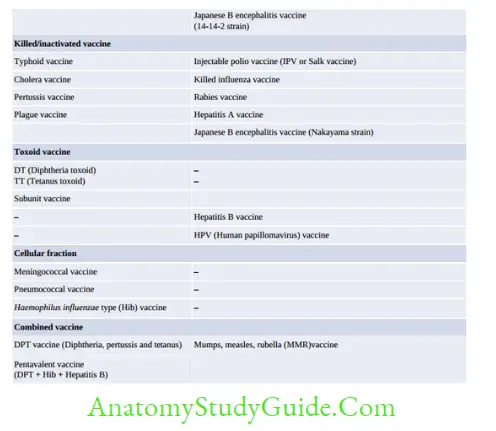

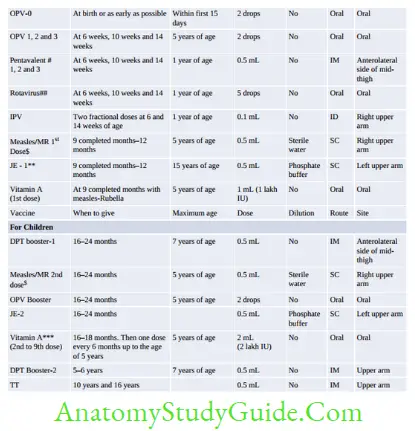

Vaccine Vial Monitor
Vaccine vial monitor is a tool to monitor the stability/potency of a vaccine and to check the efficiency of cold chain. It is heat sensitive label lining the vaccine vial. It contains an outer blue circle and an inner white square.
With time and exposure to higher temperature, the inner square changes its color gradually from white towards blue, whereas the outer circle is not heat sensitive; it remains blue throughout.


Leave a Reply