Myxoviruses And Rubella

Table of Contents
Read And Learn More: Micro Biology And Immunology Notes
Orthomyxoviruses
Influenza Virus
General Properties
- Influenza virus is spherical and possesses helical symmetry
- Viral RNA comprises of eight segments of negative-sense single-stranded RNA
- Site of RNA replication: In the nucleus (in contrast to cytoplasm by most other RNA viruses)
- Viral proteins: Influenza virus contains eight structural proteins (PB1, PB2, PA, NP, HA, NA, M1, and M2) and two nonstructural proteins (NS1 and NS2).
- Antigens and Typing
Influenza possesses two glycoprotein antigens inserted into the lipid envelope: HA (Hemagglutinin) and NA (Neuraminidase). - Hemagglutinin (HA): It binds to mucin or sialic acid receptors on RBCs, resulting in clumping of RBCs to cause hemagglutination. It also binds to the same receptors on host cells, thus facilitating viral entry. Antibody to HA is protective in nature.
- Neuraminidase (NA): It is present in fewer number. It is a sialidase enzyme that degrades the sialic acid receptors on RBCs; thus helps in:
- It displaces HA from RBCs resulting in reversal of hemagglutination called elution.
- It facilitates release of virus particles from infected cell surfaces.
- NA helps the virus to pass through the mucin layer in the respiratory tract.
Typing
- Four Genera: Based on Ribonucleoprotein (RNP) and Matrix (M) proteins:
- Influenza: A (MC cause of outbreak/epidemic, only cause of pandemic)
- Influenza B (rarely seen), Influenza C (not circulating now) and Influenza D (infects cattle and are not pathogenic to humans).
- Subtypes
- Based on HA and NA antigens, Influenza A has distinct 18 H subtypes (H1 to H18) and 11 N subtypes (N1-N11).
- Influenza B and C viruses though have subtypes; but are not designated.

Antigenic Variation
Influenza virus type A and to less extent type B undergo antigenic variation, which is of two types: antigenic shift and drift. Type C influenza virus is stable.

Clinical Features
- Incubation period: 18–72 hours
- Reservoir: Animals, birds, and humans
- MC manifestation: Mild flue like illness (URTI)
- MC complication:
- Bacterial pneumonia (S. aureus > pneumococci andH. influenza) is more common than viral pneumonia
- Reye’s syndrome: Common with Type B (fatty liver following aspirin intake).
Categorization of Seasonal Influenza A/H1N1
Ministry of Health and Family Welfare, Government of India has published the guideline on categorization of seasonal influenza A/H1N1 cases.
While screening the patients with influenza-like illness, this guideline helps in taking decision on performing laboratory test, initiating antiviral treatment or on home isolation or hospitalization.
GISRS: Influenza surveillance has been conducted globally through Global Influenza Surveillance and Response System (GISRS) under World Health Organization (WHO).
It monitors the evolution of influenza viruses globally and serves as a global alert mechanism for the emergence of pandemic influenza viruses.

Laboratory Diagnosis
- Specimen collection: Nasopharyngeal swab (polyester or Dacron swabs).
- Transported in viral transport media, kept at 4°C up to 4 days, thereafter at –70°C.
- Isolation of virus: Embryonated eggs and primary monkey kidney cell lines.
- Egg inoculation
- Amniotic cavity inoculation: It supports growth of Influenza A, B, C
- Allantoic cavity inoculation: Supports growth of only Influenza A
- Growth is detected by: Hemagglutination with Fowl and Guinea pig RBC
- Type A: Agglutinates with Guinea pig RBC, Type C: Agglutinates with fowl RBC at 4°C; and Type B: Agglutinates with both.
- Antigen detection from nasopharyngeal cells by direct IF test.
- Antibody detection: Fourfold rise in the antibody titer is more significant:
- Hemagglutination inhibition test (HAI)
- Neutralization test: It is the most specific and the best predictor of susceptibility to infection, but is time-consuming and difficult to perform.
- ELISA is more sensitive than other assays.
- Molecular methods:
- RT-PCR (reverse transcriptase polymerase chain reaction): It is highly sensitive, specific and rapid
- Real-time RT-PCR: It is currently the gold standard method for influenza diagnosis and has been recommended by Government of India. It is quantitative, useful to detect viral load in the clinical sample. Higher sensitivity and specificity than RT-PCR with turnaround time of 2–3 hours.
It simultaneously detects the three common seasonal flu strains (A/H1N1, A/H3N2 and type B).

Vaccine
Injectable Vaccines
- Types: There are 3 types of injectable vaccines:
- Inactivated influenza vaccine (IIV), e.g. Fluzone: Prepared in allantoic cavity of embryonated chick eggs and inactivated by formalin or beta propiolactone; hemagglutinin antigen content (15 μg of HA/dose).
- Cell culture-based inactivated influenza vaccine (ccIIV3); e.g. Flucelvax: prepared in cell lines such as Madin-Darby Canine Kidney (MDCK) cell line.
- Recombinant influenza vaccine (RIV); e.g. FluBlok: Contains recombinant influenza HA antigens in trivalent formulation.
- Schedule: Single dose administered by intramuscular (IM) route; except for 6 months to 8 years of age (2 doses are required ≥4 weeks apart)
- Timing of vaccination: Optimally before onset of influenza season, i.e. by end of October.
Efficacy: 25–67% (25% for H3N2, 42% against type B and 67% against H1N1). Immunity lasts for 6–12 months.
Indications: Routine annual influenza vaccination is recommended for all persons aged ≥6 months who do not have contraindications. - Contraindication: IIV should not be administered to people who have allergy to eggs or have history of hypersensitivity to previous dose of vaccine
- Travelers: If traveling to an area of increased influenza activity; vaccination, preferably ≥2weeks before departure.
Live Nasal Spray (Trivalent)
- It is trivalent: Contains influenza A (H1N1) virus, influenza A (H3N2) virus and influenza B virus.
- Stimulates both local + systemic immunity, not given if immunity is low.
- Cold-adapted strain is used.
- Indication: Recommended to all healthy persons of 2–49 years age, but is not given to high-risk groups. However, due to efficacy issues, it is not recommended for use in any population for 2017–18.
Treatment
Matrix M2 inhibitor (amantadine) and neuraminidase inhibitor (oseltamivir).
Sialic Acid Receptors Determines Pathogenicity
Influenza virus entry into the host cells is dependent on type of sialic acid receptors present on the host cell surfaces; which are specific for HA antigens of influenza.
α 2–6 sialic acid receptors are specific for human influenza strains and are found abundantly on human upper respiratory tract epithelium but not on lower upper respiratory tract.
This explains why most human flu strains cause mild upper respiratory tract infections but not pneumonia.
- α 2–3 sialic acid receptors are specific for avian influenza strains and are found abundantly on bird’s intestine.
- In humans, they are present in very few numbers on lower upper respiratory tract.
- This explains why avian flu strains cannot easily infect humans and need close contact.
However, once infected, they can infect lower respiratory tract and cause pneumonia.
Avian Flu Infection in Humans
Birds are the primary reservoir for influenza viruses.
It is believed that, to date, all human pandemic strains have originated by reassortment between avian and human influenza viruses and the mixing has occurred in pigs.
- A/H5N1 is the MC avian flu strain that has been endemic in the world.
- Origin: It was first reported from Hong Kong in 1997 and has spread to various countries including India.
- Transmission to man occurs only from birds, and requires close respiratory contact.
- Less morbidity: As there is no human-to-human transmission, morbidity is less.
- More mortality: Avian flu strains are highly virulent (due to PB1F2 protein) and mortality rate is > 60%.
- Clinical feature: H5N1 avian flu strains are associated with higher rates of pneumonia (>50%) and extrapulmonary manifestations such as diarrhea and CNS involvement.
- Other avian flu strains that can cause human infections are:
A/H7N7 (Netherlands), A/H9N2 (Hong Kong) and A/H7N9 (caused an outbreak in China, 2013) - Laboratory diagnosis: By real-time RT- PCR detecting specific HA and NA genes.
- Treatment: Drug of choice is oseltamivir (Tamiflu).
Influenza A (H1N1) pdm09
- It has caused the most recent pandemic of influenza, emerged in California in March 2009 and rapidly spread to the entire world including India, over the next few months. WHO declared the pandemic in 11th June 2009.
- Epidemiology
- Origin: H1N1 2009 flu originated by genetic reassortment of four strains (1 Human strain + 2 swine strains + 1 avian strain) and the mixing has occurred in pigs.
- Though people commonly use the word ‘swine flu’ to describe H1N1 2009 flu, but this is not the correct terminology as it is a reassortant of four strains.
- Transmission: It can be transmitted from human to human, which has accounted for its rapid spread.
- However, it is less virulent (as it lacks the PB1F2 protein). Therefore in contrast to H5N1, the H1N1 2009 flu has caused more morbidity but less mortality.
- Situation in World: Currently, World is in the post pandemic period except in India and New Zealand where still local intense transmission is ongoing.
- Situation in India:
- Between 2010–2017, about 1,15,630 cases and 8,681 deaths due to H1N1 were reported from India.
- Maharashtra was the worst-hit state with 23,958 cases and 2,710 deaths, followed by Gujarat and Rajasthan.
- Sikkim and Lakshadweep are the only two states/UTs with no cases over the past seven years.
- Varied with year: Year 2015 (maximum), 2017 and 2010 recorded highest number of cases
- The first peak occurred in 2010 (20,604 cases with 1,763 deaths)
- Second peak was observed in 2015 (threatening outbreak, 42,592 cases with 2,990 deaths). Mortality rate was higher during this season than during pandemics of 2009
- The third peak occurred in 2017 (38,811 cases with 2,266 deaths). Gujarat has the highest number of cases followed by Maharashtra; however, mortality was higher in Maharashtra.
Clinical Features
- Uncomplicated influenza: Most of the cases are present with mild URTI and diarrhea.
- Complicated/severe influenza can occur very rarely in high-risk groups, characterized by features such as secondary bacterial pneumonia, dehydration, CNS involvement, and multiorgan failure.
Laboratory Diagnosis
Real-time RT-PCR can detect and quantify the viral RNA. Diagnosis is same as described under for Influenza viruses.
Treatment
H1N1 flu is resistant to amantadine. Drug of choice is neuraminidase inhibitors:
Oseltamivir (Tamiflu) tablet: 75 mg twice a day for 5 days
- Zanamivir (10 mg, inhalational form)
Note: Matrix protein M2 inhibitors such as amantadine and rimantadine can be given for some strains of influenza A infection. However, strains of A/H1N1 2009 flu and A/H5N1 avian flu and influenza B virus have developed resistance.
Chemoprophylaxis
Chemoprophylaxis is recommended to the following groups: (1) if not vaccinated or vaccinated recently (<2 weeks), (2) HIV infected people
- Duration:
- Non-outbreak exposure (e.g. in community): It should be started as soon as possible following exposure (within 48 hours) and continued for 7 days.
- During outbreaks in hospitals (for elderly persons and children and healthcare workers): for 2 weeks, and to be continued up to 1 week after the last known case was identified.
- Antiviral drugs recommended are:
- Oseltamivir is the drug of choice. It is given as 75 mg orally, once a day for 7 days
- Zanamivir: 10 mg (two 5-mg inhalations) once daily for 7 days.
- Efficacy: 70% to 90% in preventing influenza.
Paramyxoviruses
Parainfluenza Viruses
Human parainfluenza viruses are one of the major causes of lower respiratory tract disease.
- Common cold syndrome such as rhinitis and pharyngitis is the MC presentation
- Croup (laryngotracheobronchitis): Seen with type 1 and 2 and involves older children
- Pneumonia or bronchiolitis: Occurs very rarely to 6 months, especially with type 3
- Otitis media is the MC complication of parainfluenza virus infection.
- Reinfections are common. There is no cross-protection between the serotypes.
Mumps Virus
Mumps virus is the most common cause of parotid gland enlargement in children. Transmission is through the respiratory route via droplets, saliva, and fomites. Incubation period is about 19 days (range, 7–23 days).
Clinical Manifestation
- Inapparent infection: (Most common).
- Bilateral parotitis: It is the MC manifestation (70–90%). Rarely, other salivary glands may also be involved.
- Epididymo-Orchitis is the next MC manifestation of mumps. Orchitis is unilateral in most of the cases hence, infertility is rare.
- Aseptic meningitis occurs in <10% of cases, with a male predominance. It is self-limiting condition except the deafness (due to cranial nerve palsy) which may be permanent.
- Oophoritis occurs in about 5% of women.
- Pancreatitis occurs in 4% of infections and may lead to diabetes.
- Atypical mumps: Parotitis may be absent in 10% of cases and patients are directly presented with aseptic meningitis.
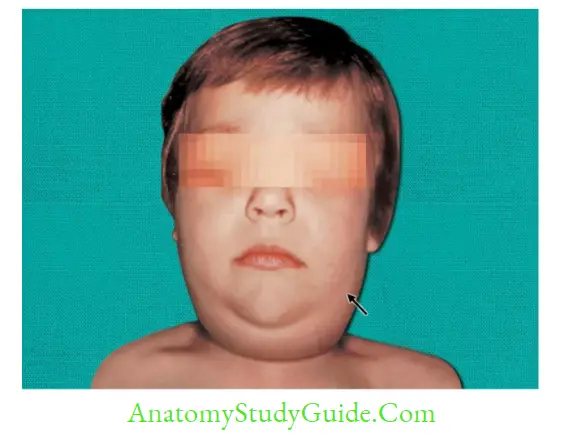
Epidemiology
Mumps is endemic worldwide, sporadic cases occurring throughout the year, with a peak in cases typically in winter and spring. Epidemics occur every 3–5 years; typically associated with unvaccinated people living in overcrowded areas.
- Period of communicability: Patients are infectious from 1 week before to 1 week after the onset of symptoms.
- Source: Cases are the source of infection. There is no carrier state.
- Reservoir: Humans are the only reservoir of infection.
- Age: Children of 5–9 years age are MC affected. Disease tends to be more severe in adults.
- Immunity: One attack (either by vaccine or infection) gives lifelong immunity.
- Secondary attack rate is high (86%).
Prevention (Live Attenuated Vaccine)
- Jeryl Lynn strain is the recommended strain used worldwide. Other strains are RIT 4385,Urabe strain and L-Zagreb.
- It is prepared in chick embryo cell line.
- Mumps vaccine is available as:
- Trivalent MMR vaccine (live attenuated measles-mumps-rubella vaccine) or
- Quadrivalent MMR-V vaccine (contains additional live attenuated varicella vaccine)
- Monovalent mumps vaccine (not commonly used)
- Schedule: Two doses of MMR is given by IM route at1 year and 4–6 year (before starting of school)
- Efficacy is about 90% after the second dose. Neutralizing antibodies appear in 95% of the recipients.
Measles (Rubeola) Virus
Measles is an acute, highly contagious childhood disease characterized by fever and respiratory symptoms, and rash.
Transmission occurs predominantly via the respiratory route.
Clinical Manifestations
Incubation period is about 10 days which may be shorter in infants and longer (up to 3 weeks) in adults
- Fever is the first manifestation, occurs on day-1 (i.e. on 10th day of infection)
- Koplik’s spots are pathognomonic of measles, appear after two days following fever and is characterized by:
- White to bluish spot (1 mm size) surrounded by an erythema
- Appear first on buccal mucosa near second lower molars, rapidly spread to entire buccal mucosa
- Rash: Maculopapular dusky red rashes appear after four days of fever (i.e. at 14th day of infection).
- Rashes appear first behind the ears → then spread to face, arm, trunk → then fade in the same order.
- Rashes are typically absent in HIV infected people.
Complications
- Secondary bacterial infections: Following measles, there is profound CMI suppression which in turn predisposes to various secondary bacterial infections.
- Otitis media and bronchopneumonia are the most common
- Worsening of underlying tuberculosis with a false negative Mantoux test
- Complications due to measles virus itself
- Giant-cell pneumonitis in immunocompromised children, and HIV-infected people
- Acute laryngotracheobronchitis (croup)
- Diarrhea, leads to malnutrition including vitamin A deficiency
- CNS complications are rare but most severe.
- Postmeasles encephalomyelitis
- Measles inclusion body encephalitis
- Subacute sclerosing panencephalitis (SSPE)-is a slowly progressive disease characterized by seizures and progressive deterioration of cognitive and motor functions.
- SSPE belongs to group C slow virus infection, caused by a defective measles virus.
- Occurrence is 1 in 300,000 measles cases
- SSPE typically occurs in persons infected with measles virus at < 2 years of age.
- SSPE usually develops after 7–10 years of initial infection.
- It is fatal within 1–3 years of onset with mortality rate of 10–20%.
- High titer antibody in CSF is diagnostic.

Laboratory Diagnosis
- Specimen: Nasopharyngeal swab
- Antigen detection: By using anti-nucleoprotein antibodies
- Virus isolation: Monkey or human kidney cells or Vero/hSLAM cell line produces cytopathic effect as multinucleated giant cells (Warthin-Finkeldey cells) containing both intranuclear and intracytoplasmic inclusion bodies.
- Antibody detection in serum: Against nucleoprotein antigen by ELISA or neutralization tests.
- Reverse-transcriptase PCR: detects viral RNA.
Live Attenuated Measles Vaccine
- Strains: All are derived from the original Edmonston strain isolated in 1954, which includes:
- Schwartz strain (currently serves as the standard in much of the world)
- Edmonston-Zagrebstrain
- Moraten strain
- Vaccine is prepared in chick embryo cell line
- Reconstitution: Vaccine is available in lyophilized form and it has to be reconstituted with distilled water and then should be used within 4 hours.
- Vaccine is thermolabile, hence it must be stored at 20°C.
- One dose (0.5 ml) containing > 1000 infective viral units is administered subcutaneously.
- Indication: Under national immunization schedule of India, measles-rubella (MR) vaccine is given at 9 completed months to 12 months along with vitamin A supplements and second dose of MR vaccine at 16–24 months. The second dose is initiated only in selected states,namely Karnataka, Tamil Nadu, Goa, Lakshadweep and Puducherry.
- Side effects include:
- Mild measles-like illness develops (15–20%). There is no spread of the vaccine virus in the community.
- Toxic shock syndrome (due to contamination of vaccine vial with S. aureus toxins).
- Measures taken following exposure
- Measles vaccine is given within 3 days of exposure. This is because incubation period of measles induced by the vaccine strain is about 7 days, compared to 10 days for the naturally occurring measles.
- Measles immunoglobulin can also be given within 3 days, at a WHO-recommended dose of 0.25 mg/kg of body weight.
- However, both should not be given together. At least 8–12 weeks of gap must be maintained.
Epidemiology
Measles is endemic throughout with epidemics recur regularly every 2–3 years, typically in late winter and early spring.
- Source: Cases are the only source of infection. There is no carrier stage.
- Reservoir: Humans are the only reservoir of infection. There is no animal reservoir.
- Period of communicability: Patients are infectious from four days before to four days after the onset of rash.
- Secondary attack rate is very high (> 90%)
- Age: Measles is a childhood disease
- Children (6 months to 3 years) in developing countries.
- Older children (> 5 years) in developed countries or in vaccinated population.
- Immunity: No age is immune if there is no previous immunity.
- There is single serotype, hence one attack (vaccine or infection) gives lifelong immunity.
- Infants are protected up to 6 months due to pre-existing maternal antibodies.
- Measles genotypes: There are 8 clades of measles which are further grouped into 23 genotypes (WHO). Globally, genotype B3 is the most common, where as in India, D8 is common.
- Epidemic of measles occurs if proportion of susceptible children exceeds 40%.
- Recent outbreaks: According to WHO, outbreaks of measles during 2016–17 have been reported from various countries such as Italy, Romania, China, Ethiopia, India, Indonesia, etc. The largest recent outbreak was from Philippines (2014, >50,000 cases). In 2017, India reported 55,226 cases (40% of total cases globally) with annual incidence of 41.7 cases per million population.
Measles Eradication
With the efficient and widespread immunization programme, it is possible to eradicate measles from the world.
WHO has introduced ‘The Strategic Plan for Measles Elimination and Rubella Control’. The target time-line for South East Asia Region (including India) is set by 2020. The following objectives are set to achieve this target:
- ≥95% coverage with two doses of MR vaccine.
- Develop and sustain a case-based surveillance system
- Develop and maintain an accredited measles and rubella laboratory network
- Strengthen support and linkages to achieve the above three strategic objectives.
Measles is said to be eliminated if the confirmed cases are <1 per million population. The American region and some of the western pacific region (Australia, Hong Kong, Japan, Korea,New Zealand, etc.) have recently achieved the measles elimination status.
Nipah Virus And Hendra Virus
They are zoonotic paramyxoviruses. Hendra virus was first isolated in 1994 in Hendra (Australia) and Nipah virus was discovered in 1999 in Malaysia.
- Reservoir: Fruit bats (flying foxes) are the natural host for both Nipah and Hendra viruses
- Geographical distribution: Human infection of Nipah virus is emerging especially in
Southeast Asia including Bangladesh (mainly), India, Thailand and Malaysia. 477 cases were reported so far with 52% mortality.- Nipah virus in India: Two outbreaks of Nipah virus in humans were reported from West Bengal (Siliguri in 2001 and Nadia in 2007); together accounted for 71 cases with 50 deaths. In 2018, cluster of 18 labaratory confirmed cases of Nipah encephalitis (with 16 deaths) have been reported from Kozhikode, Kerala.
- Transmission:
- Hendra virus is transmitted by exposure to infected body fluids and excretions of horses.
- Transmission of Nipah virus to humans may occur after direct contact with infected bats,pigs, or persons. Consumption of infected raw date palm sap is thought to be another mode of transmission.
- Clinical manifestations: Both the viruses can produce of encephalitis in humans
- Laboratory diagnosis:
- Real-time PCR from throat and nasal swabs, CSF, urine, and blood should be performed in the early stages of disease.
- Antibody detection by ELISA (IgG and IgM) in later stage.
- Immunohistochemistry on autopsy tissues -confirm the postmortem diagnosis. They are also prone to cause laboratory-acquired infections and are classified as biosafety level 4 pathogens.
- Treatment: No antiviral drug is available.
Respiratory Syncytial Virus
Clinical Manifestations
Infants: RSV is the most common cause of lower respiratory tract infection below 1 year of age, causing bronchiolitis, pneumonia, and tracheobronchitis.
- Adults: RSV produces influenza-like upper respiratory symptoms.
- RSV can cause exacerbation and worsening of asthma or COPD.
- Recurrent infection is common, but is much milder (common cold).
Laboratory Diagnosis
- Virus isolation: HeLa and HEp-2 are the most sensitive cell lines for virus isolation.
- A characteristic cytopathic effect, syncytium formation (multinucleated giant cell) appear after 10 days, hence it is named as syncytial virus.
Epidemiology
- Seasonality: Rainfall, in winter and spring.
- Age: Infants between ages of 6 weeks to 6 months of age.
- Subgroups: RSV can be typed into two subgroups; Subgroup A infections appear to cause more severe illness.
Treatment
- Ribavirin is the drug of choice. It is indicated for severe infections in infants. However,its beneficial effect to older children and adult is doubtful. It is administered as aerosol for 3–6 days.
Rubella
Rubella is not a myxovirus, but discussed here because of its clinical overlapping with measles.
Rubella is also known as German measles. It belongs to family Togaviridae.
Epidemiology
- Source: Only cases, No carriers
- Once infected: Provides lifelong immunity
- In India, still 40% females of reproductive age group are susceptible to rubella infection.
- Period of communicability: 1 week to +1 week of rash
- IP-2–3 weeks (14 days)
- Transmission: Droplet, contact, sexual, in-utero.
Clinical Manifestations in Adult
- Subclinical infections: 50%
- Rash on day 1 (face): lasts for 3 days
- Lymphadenopathy (occipital and postauricular)
- Forschheimer spots: Pin-head sized petechiae seen on the soft palate and uvula. They appear with onset of rash.
Congenital Rubella Syndrome
Risk of transmission and severity is maximum in 1st trimester of pregnancy, after 5th month: risk is negligible
- Permanent congenital defects
- Classical Triad:
- Ear defect: Nerve deafness (MC defect of congenital rubella syndrome)
- Ocular defects: Salt-and-pepper retinopathy is the MC ocular defect followed by cataract
- Cardiac defect: Patent ductus arteriosus (PDA) is the MC cardiac defect followed by pulmonary artery stenosis and ventricular septal defect.
- CNS defects such as microcephaly and mental retardation, and motor delay and autism
- Classical Triad:
- Transient congenital changes such as hepatosplenomegaly, bone lesion, intrauterine growth retardation (IUGR) and thrombocytopenia with petechiae (Blueberry muffin syndrome) may be seen.
Laboratory Diagnosis
- Viral Isolation:
- Ideal specimen: Nasopharyngeal or throat swabs
- Ideal cell line: Monkey or rabbit origin cell lines may be used.
- Identified by interference with Echovirus
- Serology (Antibody detection): Hemagglutination inhibition test (HAI) and ELISA (widely used). Interpretation of serology in congenital rubella infection:
- IgM antibodies do not cross placenta; their presence in a neonate is diagnostic of congenital rubella infection.
- IgG antibodies cannot differentiate between maternal transfer and a true congenital infection. However, IgG persisting in baby’s serum beyond the expected time of disappearance of maternal IgG (9 months of age) can also be used for diagnosis.
Rubella Vaccination (RA 27/3 Live Attenuated)
- Prepared from Human diploid cell line, Single dose (0.5 ml) of vaccine is administered subcutaneously.
- Following vaccination, seroconversion occurs in 90% of recipients and immunity persists for 14–16 years or probably lifelong.
- Vaccine is contraindicated in pregnancy.
- As it is teratogenic, pregnancy should be avoided at least for 4 weeks (28 days) following vaccination.
- Infants below 1 year should not be vaccinated due to possible interference from persisting maternal antibody.
- Priority groups for rubella vaccine in India: indicated in all women of reproductive age (first priority group) followed by all children (1–14 years).
- Under national immunization schedule, rubella vaccine is given along with measles (MR vaccine) at 9–12 months of age and second dose at 16–24 months at selected states.
Daywise Appearance of Rashes

Vaccine Storage
- Deep Freezer: Polio, Measles (–20°C)
- Vaccine is stored at cold part (4°C) and never allowed to freeze: DPT, Typhoid, TT, DT, BCG diluents
- Vitamin A: Outside, at room temperature
- Most of the vaccine can be stored up to 5 weeks in refrigerator: between 4–80°C
- Open multidose vials should be discarded:
- Within 1 hr (if no preservative is added, e.g. most live vaccines)
- Within 3 hr or till end of session (if preservative is added).
Leave a Reply