General Characteristics Of Nematodes
- Unsegmented, elongated, and cylindrical
- Separate sexes
- Buccal capsule present
- GIT is complete
- The body cavity is present
- Size:
- Small: Trichinella, Strongyloides, Hookworm, Trichuris
- Large: Dracanculus, Ascaris
- The body is covered with a tough cuticle.
Read And Learn More: Micro Biology And Immunology Notes
Table of Contents
Classification According to Nematodes Habitat
- Intestinal human nematodes:
- Small Intestine: Ascaris lumbricoides, Hookworm (Ancylostoma and Necator), Strongyloides
- Large intestine: Trichuris and Enterobius
- Somatic human nematodes: Filarial worm, Trichinella spiralis, Dracunculus medinensis (Guinea worm).
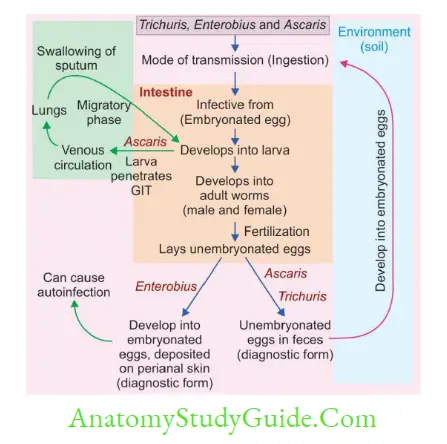
- Classification According to Whether They Produce Egg/Larva
- Viviparous: Lay Larva—Filarial worm, Trichinella and Dracunculus
- Oviparous: Lay eggs that hatch out to larva later in the environment—Ascaris, Hookworm, Trichuris, and Enterobius
- Ovoviviparous: Lay eggs containing larvae that immediately hatch out, e.g. Strongyloides.

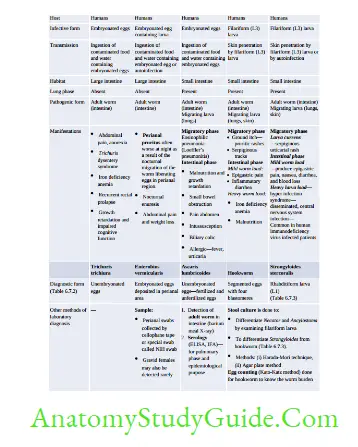
Intestinal Nematodes
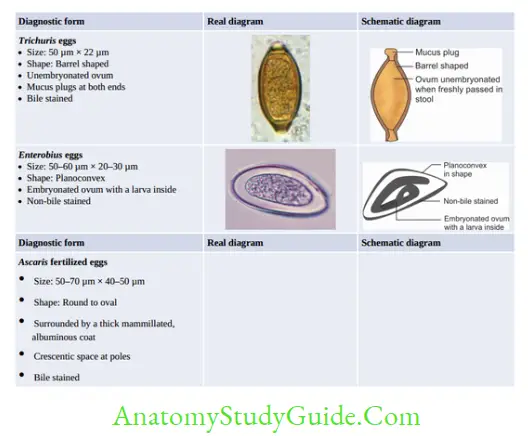
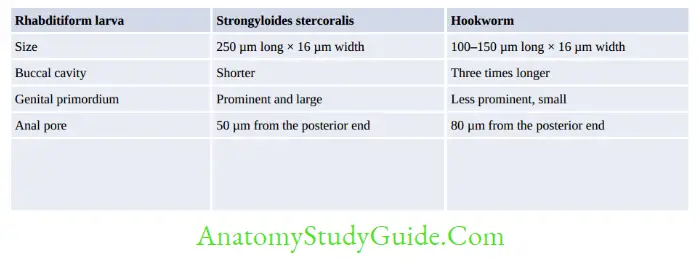
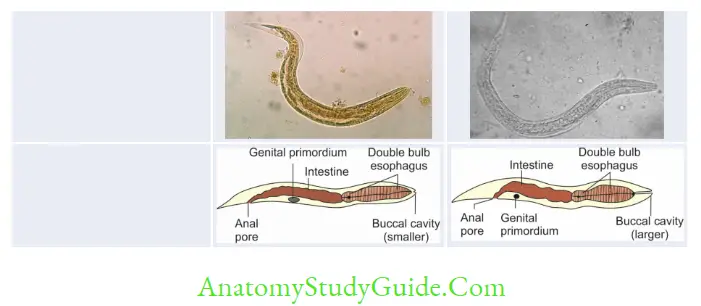
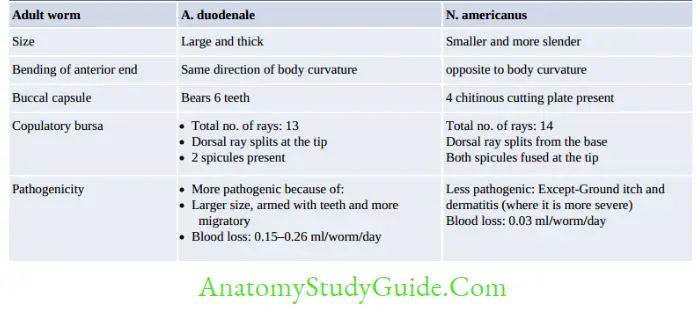
Hookworm (Necator and Ancylostoma)
- Egg and Rhabditiform larva—same
- They differ only in filariform larvae and in adult worm
- Filariform larva of Necator:
- Gap between esophagus and intestine
- Cuticle: Bears prominent transverse striation
- Buccal capsule: Larger (15 µm), lumen short.
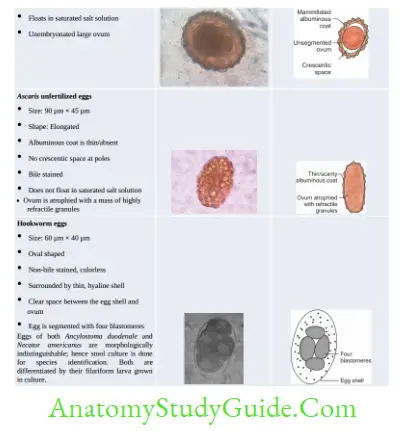
- Laboratory Diagnosis of Hookworm
- Stool microscopy—detects non-bile stained oval segmented and non-bile-stained eggs with 4– 32 blastomeres. Eggs of Acylostoma and Necator are indistinguishable.
- Stool culture—eggs develop into filariform larvae, which help in differentiating Acylostoma from Necator
- Harada-Mori filter paper tube method
- Petri dish (slant culture) technique
- Baermann funnel techniue
- Charcoal culture method
- Agar plate technique (more sensitive)
- Molecular method—detects genes such as mitochondrial cytochrome oxidase I gene, ITS-1
and ITS-2 regions of ribosomal DNA - Other findings— hypochromic microcytic anemia.
- Chandler’s index is done for hookworm: eggs per gram of stool:
- Below 200: Hookworm is not of much significance
- 200–250: May be regarded as a potential danger
- 250–300: Minor public health problem
- Above 300: Important public health problem
- N. americanus is the predominant hookworm in India (and the world) and except in Punjab and UP
(Ancylostoma is more common)
Wakana disease:
- Seen only in Ancylostoma but not in Necator; as their L3 stage fails to develop after ingestion.
- Occurs: When L3 larva is transmitted by oral route (Not by skin penetration)
- Common symptoms include nausea, vomiting, pharyngeal irritation (GIT symptoms), cough, dyspnea, and hoarseness (pulmonary symptoms).
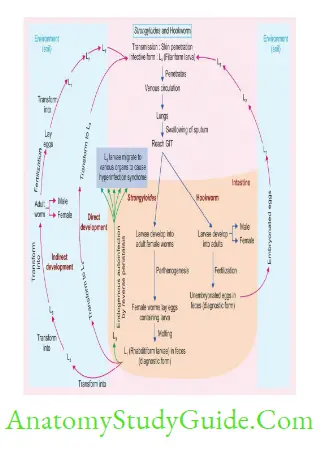
Strongyloides Stercoralis
- Parthenogenesis: Female Strongyloides worms can directly lay eggs without fertilization; by a process by which the females produce offspring without fertilization with males.
- Hyperinfection syndrome: Autoinfection is responsible for maintaining the infection as long as 30–40 years and can cause disseminated infection and hyperinfection syndrome.
- The underlying cause of hyperinfection syndrome is the repeated autoinfection cycles; which leads to the generation of a large number of filariform larvae. The larvae penetrate the GIT and migrate to various organs.
- Risk factors: Impaired host immunity, Glucocorticoid therapy, immunosuppressive conditions such as transplant recipients, hematologic malignancies, and intake of immunosuppressive drugs.
- hyperinfection syndrome is common in patients coinfected with human T cell lymphotropic virus type (HTLV-1)
- Coinfection of Strongyloides with HIV is common. However, it is not associated with disseminated strongyloidiasis.
- Clinical Features: Colitis, enteritis, or malabsorption, and in severe cases disseminated strongyloidiasis may develop
Disseminated strongyloidiasis:
- Larvae may invade the GIT and migrate to various organs including CNS, peritoneum, liver, and kidneys
- Passage of enteric flora through disrupted mucosa leads to gram-negative bacterial sepsis,
pneumonia, or meningitis - CNS invasion, brain abscess, and meningitis are common; larvae may be seen in CSF.
- Eosinophilia is often absent in severely infected patients
- The mortality rate in untreated patients approaches 100% and even with treatment, it may exceed 25%.
Laboratory Diagnosis
- Microscopy [stool or duodenal aspirate (by Entero-test), rarely sputum]—detects rhabditiform larvae
- Stool culture—Same as for hookworm
- Antibody detection—ELISA (CrAg-ELISA), luciferase immunoprecipitation assay
- Coproantigen in stool—capture ELISA detecting excretory/secretory (E/S) antigen
- Molecular diagnosis—real-time PCR detecting cytochrome C oxidase subunit I gene, 18S rRNA, or 28S RNA gene sequences.
Strongyloides fuelleborni
It causes Swollen belly syndrome: A serious life-threatening condition characterized by diarrhea, respiratory distress and protein-losing enteropathy, leading to hypoalbuminemia and edema
- S. fuelleborni infection in humans is seen in tropical forest regions of Central and East Africa. It is zoonotic in these areas.
- A non-zoonotic infection occurs in forest areas of Western Papua New Guinea; affecting infants of 2–4 months of age.
It is possibly transmitted through breast milk. - Diagnosis: It is diagnosed by detecting the eggs (50–70 μm long) but not larvae in the stool (different from S. stercoralis).
- Treatment: Thiabendazole is DOC.
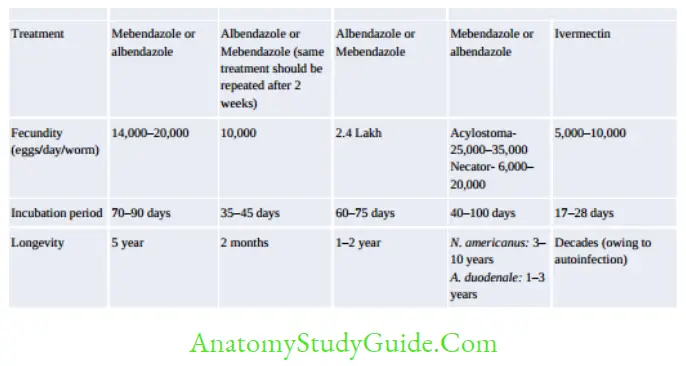
Treatment
- Albendazole is the DOC of all nematodes except
- Enterobius: Mebendazole
- Strongyloides: Ivermectin
- Wuchereria and Brugia: DEC
- Onchocerca: Ivermectin
- Dracunculus: Metronidazole.
Filarial Nematodes
Pathogenesis and Life Cycle
- Infective form: Filarial larvae (L3) transmitted by Mosquito bite
- L3 larvae migrate through lymphatics to local LN where they transform into adult worms and then to L1(microfilaria)
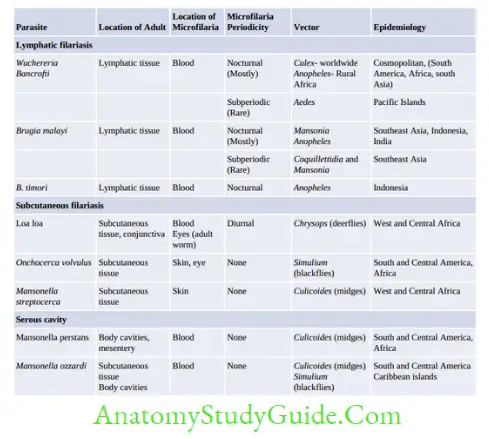
- Epidemiology: W. bancrofti, is the most widely distributed filarial parasite of humans.
- Southeast Asia accounts for the highest-burden and comprises 50% of globally infected lymphatic filariasis (LF) cases.
- Globally, 90% of lymphatic filariasis is caused by Wuchereria bancrofti and the remainder by Brugia species, and in India, the ratio is 99.4 and 0.6% respectively.
- In India: Highly endemic states are Uttar Pradesh, Jharkhand, Bihar, and West Bengal, which account for two-thirds of the lymphatic filariasis burden in India
- Prevalence is low in northeastern states, Jammu Kashmir, and Punjab.
- Subperiodic W. bancrofti (transmitted by Aedes) has been reported from Nicobar Island.
- Microfilaria are not pathogenic but are periodically discharged to blood (diagnostic form)
- Adult female worm: Crucial role in pathogenesis
- Triad of pathogenesis:
- Dilatation of lymphatic vessel
- Lymphadenitis
- Obstruction to lymphnode: Fibrotic degeneration of lymph vessels.
- Endosymbiosis: Pathogenic W. bancrofti is found to be infected with a Rickettsia group of bacteria called Wolbachia and maintains an endosymbiotic relationship. It is proved that this symbiosis is essential for the parasite’s survival, fertility, and larval development.
- Age and gender: Microfilaremia increases with age; starts at 5 years and peaks at >30 years.
- Males are more commonly affected than females.
Lymphatic Filariasis
- Endemic Normal:
- Asymptomatic, no microfilaria in the blood
- Occurs due to insufficient exposure, immunological resistance, and prepatent period at the time of detection.
- Asymptomatic Stage:
- Microfilaria present in blood, but no clinical feature
- Th1 is down-regulated but Th2 is high (IL4↑ )
- After several years, hyporesponsiveness breaks, and an inflammatory reaction occurs.
- Acute Filariasis:
- Due to antigens released from female adult worm
- Filarial fever: High-grade fever
- Lymphatic inflammation (lymphangitis and lymphadenitis)
- Transient local edema
- Dermatolymphangitis
- Chronic Filariasis:
- 10–15 years after the acute phase
- Fibrotic changes occur (obstructive phase) in lymph vessels
- Featured by:
- Lymph varies
- Hydrocele
- Elephantiasis of the scrotum, leg, arms, breast, and vulva (nonpitting edema)
- Granuloma of female breast
- Chyluria chyle in urine (d/t obstruction of lymph vessels of kidney and abdomen).
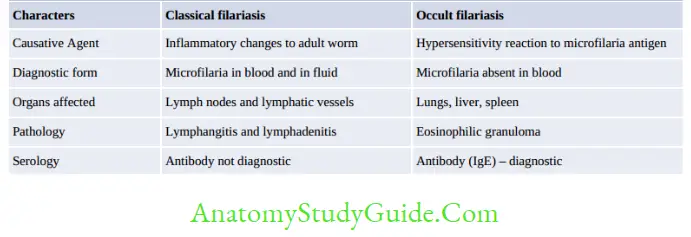
- Occult Filariasis or Tropical pulmonary eosinophilia (TPE)
- Also called Weingarten’s syndrome or Meyers-Kouwenaar Syndrome.
- It develops in some infected individuals of endemic places.
- It represents a hypersensitivity reaction to microfilaria antigen.
- Microfilariae are rapidly cleared from the bloodstream and filtered, lodged, and destroyed in the lungs initiating an allergic response.
- Hence, microfilariae are not detected in peripheral blood.
- Epidemiology: The majority of cases have been reported from India, Pakistan, Sri Lanka,
Brazil, Guyana, and Southeast Asia. - Males are affected more than females (4:1), mainly in the third decade of life.
- Clinical features: nocturnal paroxysmal cough and wheezing weight loss, low-grade fever.
- Occasionally, microfilariae are entrapped in other organs like the spleen, liver, and lymph node leading to hepatosplenomegaly and lymphadenopathy.
- Diagnosed by: increased blood eosinophilia (absolute eosinophil count > 3000/μL), diffuse infiltration in chest X-ray and elevated serum IgE levels.
- Treatment: DEC for 14 days.
Brugia Malayi
- Transmission:
- Nocturnal strains: Transmitted from Man-Man by bite of Mansonia
- Subperiodic strains: Transmitted from monkey (Zoonotic): by Mansonia
- Anopheles and Aedes rarely transmit.
Epidemiology:
- B. malayi occurs primarily in eastern India, Indonesia, Malaysia, Thailand, and the Philippines
- It also shows two types of periodicity of microfilaremia. The nocturnal form is more common.
- In India, the major states involved are Kerala, Odisha, Assam, and West Bengal.
- Pistia stratiotes plant: Important for the survival of Mansonia
- Clinical feature:
- Leg below knee: ONLY affected (Contour of the knee- normal)
- Genital involvement and chyluria do not occur.
- MC-acute adenolymphangitis and filarial abscesses.
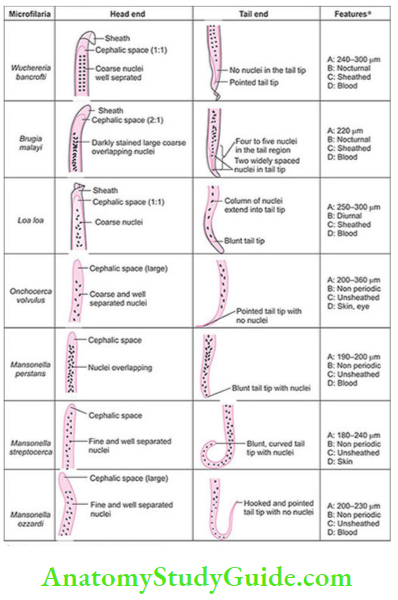
- Diagnosis: Microfilaria is differentiated from that of W. bancrofti by the pointed tail tip, Nuclei
column is darkly stained, large, coarse, overlapping, and extended till the tail tip. - B. timori: Timor island of Indonesia, Vector-Anopheles barbirostris.
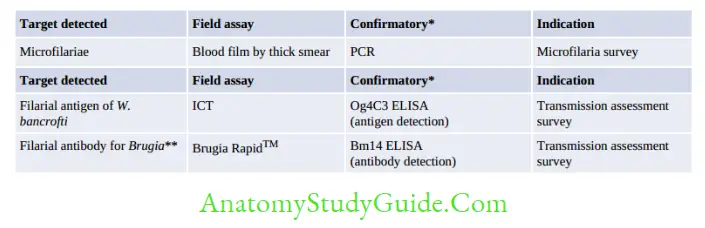
Lab Diagnosis
1. Blood microscopy:
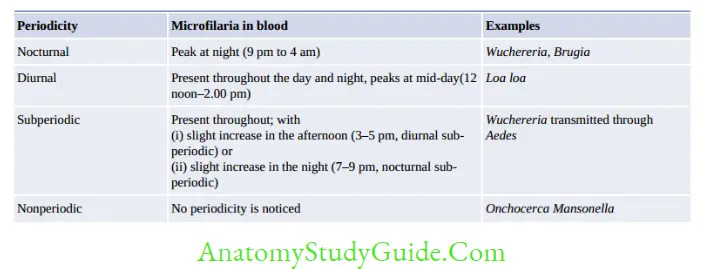
- Blood is collected during:
- Nocturnal: 10 pm to 4 am
- Subperiodic nocturnal: 8 pm to 10 pm
- Subperiodic diurnal: 2 pm to 6 pm.
- DEC provocation test: Done to demonstrate microfilaria in the daytime
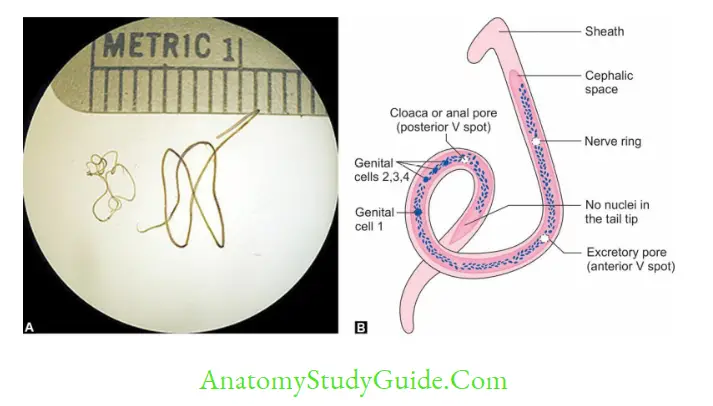
- Direct wet mount: To see the serpentine movement of microfilaria
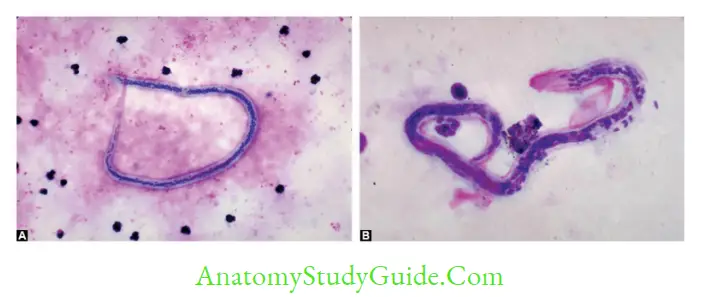
- Staining: Thick blood smear stained with Leishman/Giemsa
- W. bancrofti—tail tip pointed, free of nuclei
- B. malayi—tail tip blunt, nuclei extended upto tail tip
- Concentration methods: Membrane filtration technique and Knott’s centrifugation technique
- QBC: Quantitative Buffy Coat
- Microfilaria NOT found in peripheral blood:
- Occult filariasis
- Chronic filariasis (some cases)
- Wrong blood sampling time.
- Other samples:
- Urine microscopy: 10–20 ml early morning chylous urine
- Hydrocele fluid and LN aspirate microscopy.
2. Demonstration of antibody:
- Flow-through assay using WbSXP-1 Ag and Luciferase immunoprecipitation system using Wb123 Ag.
- Disadvantage:
- Cross-reactivity
- Unable to discriminate between recent and past infection.
3. Demonstration of antigen:
- Indicates recent infection
- More sensitive than microscopy
- Can be detected in daytime
- Can differentiate current and past infection: Antigen disappears after clinical cure.
- Can detect in urine antigen
- ELISA: Using monoclonal antibody against AD12 antigen detects adult worms only
- ELISA: Using monoclonal antibody against Og4C3 antigen detects adult worm and microfilaria
- No antigen detection methods are available for Brugia infection.
4. Molecular methods—real-time PCR detecting genes such as SspI repeat, pWb12 repeat, pWb-35, etc.
5. Imaging methods:
- X-ray: Dead and calcified worm in LN and chest X-ray shows pulmonary infiltrate in
TOP
- Ultrasound of scrotum: Live adult worms with serpentine movement (Filarial Dance sign).
6. Other methods—eosinophilia, elevated IgE.
Treatment
- Diethyl Carbamazine (DEC): DOC
- DEC + Albendazole regimen: In India
- DEC + Ivermectin regimen: In Africa
- DEC acts on both microfilaria and adult whereas ivermectin acts only on microfilaria.
- Doxycycline: It is given to target the intracellular Wolbachia.
Loa Loa
- Epidemiology: Restricted to the rain forests of West and Central Africa.
- Pathogenesis and clinical feature
- Calabar swellings: This is the most common form of loiasis, also called as fugitive swelling.
- It is a subcutaneous swelling developing on the extremities (knee or wrist) and less frequently at other sites.
- It occurs due to the host’s inflammatory response to the migrating adult worm (at a speed of 1 cm/minute)
- Microfilariae are not pathogenic.
Ocular manifestations: Conjunctival granuloma, edema of the eyelid, leading to proptosis (bulging). - Meningoencephalitis (severe complication).
Laboratory diagnosis
- Detection of microfilariae in the peripheral blood
- Isolation of the adult worm from the eye or biopsy of subcutaneous swelling
- Molecular methods: Nested PCR-based assays for the detection of L. loa DNA.
- Antibody detection: Lateral flow assay (ICT) for detection of antibodies to Ll-SXP-1 antigen.
- Treatment: Diethylcarbamazine (DEC) is the drug of choice—multiple courses are necessary to resolve loiasis completely.
Onchocerca Volvulus
- West Africa
- Skin manifestations:
- Dermatitis (Sowda )
- Leopard skin
- Onchocercoma (subcutaneous nodules).
- Ocular involvement:
- River blindness
- Punctate keratitis
- Sclerosing keratitis.
- Lymph nodes: Hanging groin
- Detection of the microfilariae: Skin snips technique
- Mazzotti skin test (DEC patch test)
- Ivermectin: DOC.
Trichinella Spiralis
- Host: Pig is the optimum host and reservoir, man is an accidental host and acts as a dead end.
- Human trichinellosis is widely prevalent in the pork eating countries like Europe, South
America and North America including the USA. - In India, human infection is very rare. The first case was reported from the Punjab. In 2010, an
outbreak of human trichinellosis had occurred in Uttarakhand affecting 18 people eating
roasted wild boar meat called kachmoli. - Infective stage: First stage (L1) larva
- Mode of transmission: By ingestion of raw or uncooked pork
- Larva penetrate the intestine and migrate to muscle where it undergo encystment
- MC muscle: Extraocular muscles followed by the biceps; and the muscles of the jaw, neck
- Diagnosis:
- Demonstration of larvae in muscle biopsy taken near tendon insertions of deltoid by
histopathologic study. - Antibody detection: Confirms the diagnosis but cannot differentiate past and present
infection. - Coproantigen detection: double sandwich ELISA to detect larval somatic antigens in
stool. - Animal inoculation: Rats are fed with muscle tissue of suspected patients and examined for T. spiralis larvae in the diaphragm.
- Other tests: Blood eosinophilia, elevated muscle enzymes, and X-ray to detect the calcified muscle cyst.
- Bachman intradermal test: Persists for life, hence cannot differentiate past and present infection.
- Demonstration of larvae in muscle biopsy taken near tendon insertions of deltoid by
- Treatment: Mebendazole and albendazole (active against the enteric stage of the parasite, Symptomatic treatment, and Glucocorticoid severe myositis and myocarditis).
Dracunculus Medinensis
- Causes Guinea worm disease or dracunculiasis
- It is eliminated from India since 2000 (and also from Pakistan), incidence has been reduced in Asia
- In 2017, only 30 cases were reported, 15 each from Chad and Ethiopia. Mali reported zero human cases for two consecutive years
- Host: Man is the definitive host and Copepods (Cyclops) is the intermediate host.
- Infective form: Third stage filariform larvae
- Mode of transmission: Drinking fresh water from stagnant pools containing minute freshwater crustaceans (Cyclops) infected with L3 larvae
- Presentation: Starts as a painful papule , becomes a blister from which the worm emerges
- Seasonal (June–September); Disease is a strong indicator of poor socioeconomic development
- Microscopic detection of L1 larvae and adults: On contact with cold water placed on the leg ulcer
- Treatment: Worm removal and symptomatic treatment
- Reasons for Eradication of Guinea worm disease from India:
- Provision of safe drinking water: Filtration of drinking water, installing hand pumps and pipes
- Cyclops control: Killing copepods in sources of drinking water by application of abate larvicide
- Provision of clean drinking water from boreholes or wells
- Health education about boiling or filtering of drinking water
- Treatment of cases.
Larva Migrans
- The life cycle of most of human nematodes involves penetration of the skin by the larval stage followed by migration of the larvae to the intestine, lungs or other organs.
- However, the larvae of lower animal nematodes when accidentally infect man, are not able to complete their normal development (because humans are an unusual host for them) and their life cycle gets arrested. The larvae wander around the aimless way in the body. This is called larva migrans.
- Two types of larva migrans exist:
- Cutaneous larva migrans: Also called as creeping eruption. Larva migration occurs in the skin and subcutaneous tissue.
- Visceral larva migrans: Larva migration takes place in the viscera.
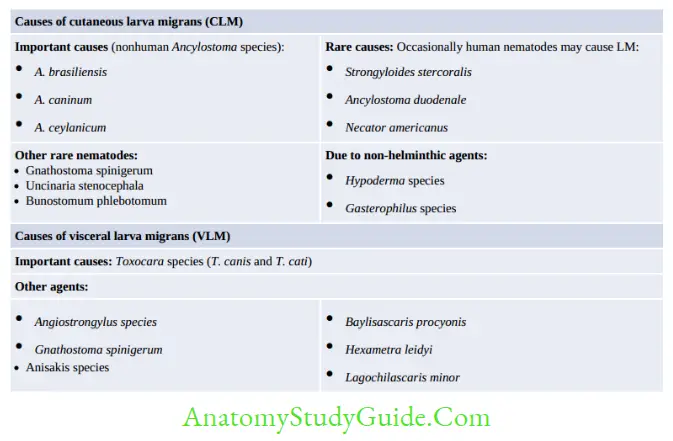
Miscellaneous Topics
- Capillaria philippinensis: Eggs resemble Trichuris
- Trichostrongylus spp. (Pseudo hookworm): Eggs resemble to that of hookworms. The rhabditiform larva’s tail end has a bead-like swelling.
- Angiostrongylus species infecting man are A. cantonensis (eosinophilic meningitis), A. costaricensis (abdominal angiostrongyliasis).
- Parasites causing malignancy
- Schistosoma haematobium: Squamous cell carcinoma of the urinary bladder
- Clonorchis sinensis: cholangiocarcinoma of the liver, bile duct, and Adenocarcinoma of the pancreas
- Opisthorchis viverrini: Cholangiocarcinoma of the bile duct.
- Parasites causing anemia
- Hookworm: Iron deficiency anemia (thrives on Plasma): Necator: 0.03 ml/day, Acylostoma: 0.2 ml/day
- Babesia: Hemolytic anemia
- Plasmodium spp.: Autoimmune hemolytic anemia
- Trichuris trichiura: Iron deficiency anemia
- Leishmania donovani: Autoimmune hemolytic anemia
- Diphyllobothrium latum: Vit. B12 Deficiency/Megaloblastic anemia.
- Parasites causing dysentery
- Entamoeba histolytica
- Balantidium coli
- Schistosoma japonicum
- Schistosoma mansoni
- Trichuris trichiura
- Soil-Transmitted Helminths
- Ascaris lumbricoides
- Ancystoma duodenal
- Ancylostoma braziliense
- Ancylostoma caninum
- Trichuris trichiura
- Strongyloides stercoralis
- Vector-borne parasitic diseases
- Plasmodium spp.
- Leishmania donovani
- Wuchereria bancrofti
- Brugia malayi
- Onchocerca volvulus
- Loa loa
- Mansonella tzaddik
- Trypanosoma brucei
- Trypanosoma cruzi
- Non-bile stained eggs (Abbreviation—NEHA)
- Necator americanus
- Enterobius vermicularis
- Hymenolepis nana
- Ancylostoma
- Autoinfection is seen in (Abbreviation—CHEST)
- Cryptosporidium
- Hymenlepis nana
- Enterobius
- Strongyloides
- Taenia solium
- Does not float in saturated salt solution (Abbreviation—ULTO)
- Unfertilized egg of Ascaris
- Larva of Strongyloides
- Taenia egg
- Operculated egg of trematodes.
Leave a Reply