Neoplasia
Question 1. Define Neoplasia.
Answer:
Definition (pre-molecular era): Abnormal mass of tissue, the growth of which exceeds and is uncoordinated with that of the normal tissues and persists in the same excessive manner after cessation of the stimuli which evoked the change
Definition (molecular era): Disorder of cell growth that is triggered by a series of acquired mutations affecting a single cell and its clonal progeny.
Read and Learn More Preparatory Manual of Pathology Question and Answers
Question 2. Differences between benign and malignant tumors.
Answer:
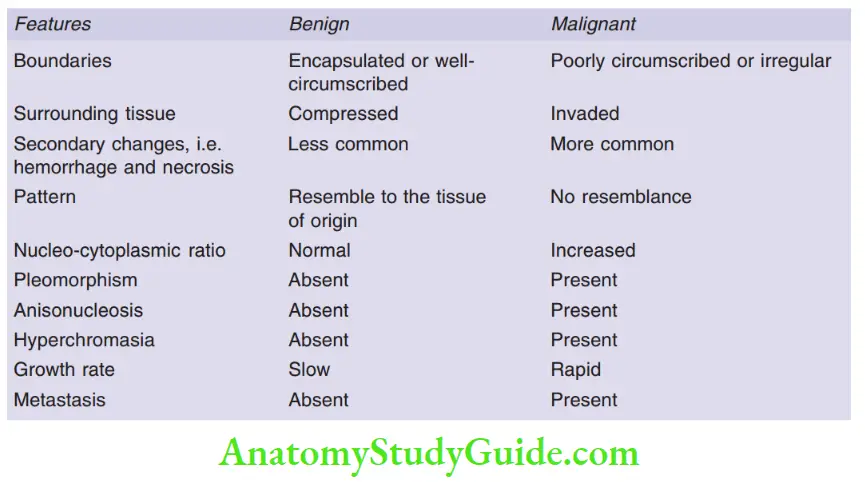
Question 3. Define metaplasia with examples.
Answer:
Metaplasia
- It is defined as the replacement of one type of cell with another type
- It is found in association with tissue damage, repair, and regeneration
- Examples: Gastroesophageal reflux damages the squamous epithelium of the esophagus, replacing it with the intestinal epithelium
Question 4. What is hamartoma?
Answer:
Hamartomas
- Are benign masses composed of cells indigenous to the involved site
- For example, hamartomatous polyps are seen in the gastrointestinal tract
Question 5. Describe the terms differentiation and anaplasia.
Answer:
Differentiation
- The extent to which the neoplastic parenchymal cells resemble the corresponding normal parenchymal cells, both morphologically and functionally
- Anaplasia: Means lack differentiation, which is a hallmark of malignancy, and is often associated with:
- Pleomorphism: Variation in size and shape of cells
- Abnormal nuclear morphology: High nuclear: Cytoplasmic ratio and hyperchromasia
- Mitoses: Atypical, bizarre mitotic figures
- Loss of polarity: Tumor cell orientation is disturbed
Question 6. What is metastasis? Discuss in brief the different pathways of the spread of cancer.
Answer:
Metastasis
- It is defined by the spread of the tumor to the sites that are physically discontinuous with the primary tumor
- It is the most reliable feature which differentiates malignant from benign tumors
- All tumors can metastasize except gliomas and basal cell carcinomas
Pathways of spread
Dissemination of cancers may occur through one of three pathways:
- Direct seeding of body cavities or surfaces,
- lymphatic spread, and
- hematogenous spread
1. Seeding of body cavities and surfaces:
- Malignant neoplasm penetrates into surrounding tissues
- Most often involves the peritoneal cavity, pleural cavity, pericardial cavity, subarachnoid and joint spaces
- For example, mucus-secreting appendiceal carcinomas fill the peritoneal cavity and are termed pseudonyms peritonei
2. Lymphatic spread:
- The most common pathway for the initial dissemination of carcinomas
- Sentinel lymph node: Defined as “the first node in a regional lymphatic basin that receives lymph flow from the primary tumor
- In the breast, a sentinel lymph node biopsy is performed before undergoing an axillary lymph node dissection
3. Hematogenous spread
- Seen most commonly in sarcomas
- The liver and lungs are most frequently involved in hematogenous dissemination
- Cancers arising in close proximity to the vertebral column embolize through the paravertebral plexus example vertebral metastases of carcinomas in the thyroid and prostate
- Renal cell carcinoma invades the branches of the renal vein
- Hepatocellular carcinomas often penetrate portal and hepatic veins
Points to remember
- Breast carcinoma spreads to the bone
- Bronchogenic carcinomas involve the adrenals and the brain
- Neuroblastomas spread to the liver and bones
Question 7. Write in detail about the molecular basis of cancers.
Answer:
Molecular basis of cancer
1. Nonlethal genetic damage lies at the heart of carcinogenesis
- Initiation of cell injury, whether induced by germline or sporadic mutation leads to genetic damage in the cell, in response to which the cell’s genetic makeup is altered, but cell death does not occur.
2. Tumor is formed by the clonal expansion of a single precursor cell that has incurred genetic damage
- Genetic mutations brought about in a cell are heritable and are passed on to all subsets of tumor cells, hence tumors are clonal
3. Cancer-causing mutations affect
- Four classes of normal regulatory genes—growth-promoting proto-oncogenes, growth-inhibiting tumor suppressor genes, genes that regulate programmed cell death (apoptosis), and genes involved in DNA repair
4. Carcinogenesis results from the accumulation of complementary mutations in a stepwise fashion over time
- Mutations that contribute to the development of malignant phenotype are referred to as driver mutations
- The first driver mutation is the initiating mutation, in which the “initiated” cell acquires a number of additional driver mutations, which result in the development of the cancer
5. In addition to tumor mutations, epigenetic aberrations (DNA methylation and histone modifications) contributes to tumor development
- Epigenetic changes are potentially reversible by drugs that inhibit DNA- or histone-modifying factors
Question 8. Write about oncogenes and their mode of activation with an example.
Answer:
Oncogenes
- Genes that promote autonomous cell growth in cancer cells
- Created by the mutations in proto-oncogenes
- Encode proteins called oncoproteins
- Oncoproteins have the ability to promote cell growth in the absence of normal growth-promoting signals
- Proto-oncogenes play a role in signaling pathways that drive proliferation
- Pro-growth proto-oncogenes encode growth factors, growth factor receptors, signal transducers, transcription factors, or cell cycle components
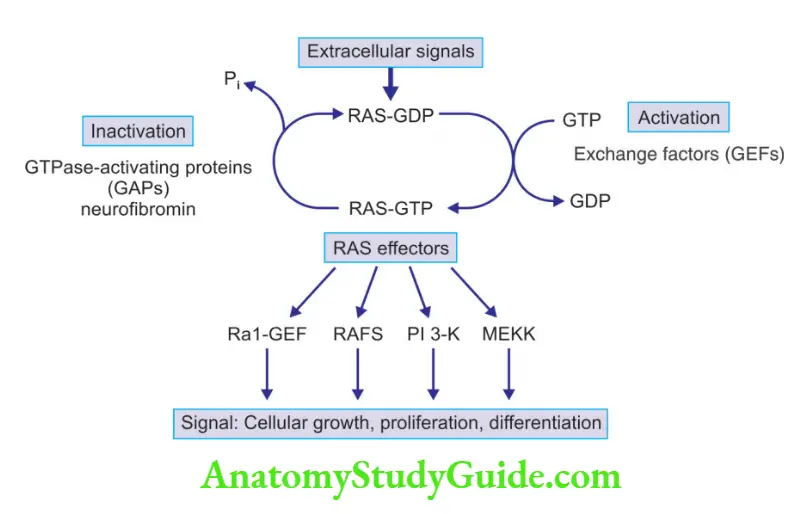
Mode of activation of RAS proto-oncogene and its effects
RAS Mutations
- The most common type of abnormality involving proto-oncogenes in human tumors
- Comprises HRAS, KRAS, NRAS
Normal pathway
- RAS binds guanosine nucleotides (guanosine triphosphate, GTP, and guanosine diphosphate, GDP)
- Inactive RAS binds to GDP
- GDP conversion into GTP activates RAS protein
- Activated RAS stimulates mitogen-activated protein (MAP) kinase cascade, and PI3K pathway, which signals cell proliferation
- GTP hydrolysis converts the GTP-bound, active RAS to the GDP-bound, inactive form
- GTPase activating proteins (GAPs): Increases the GTPase activity, leading to termination of signal transduction
- GAPs function as “brakes” that prevent uncontrolled RAS activity
- Mutation in neurofibromin-1, a GAP, is associated with familial neurofibromatosis type 1
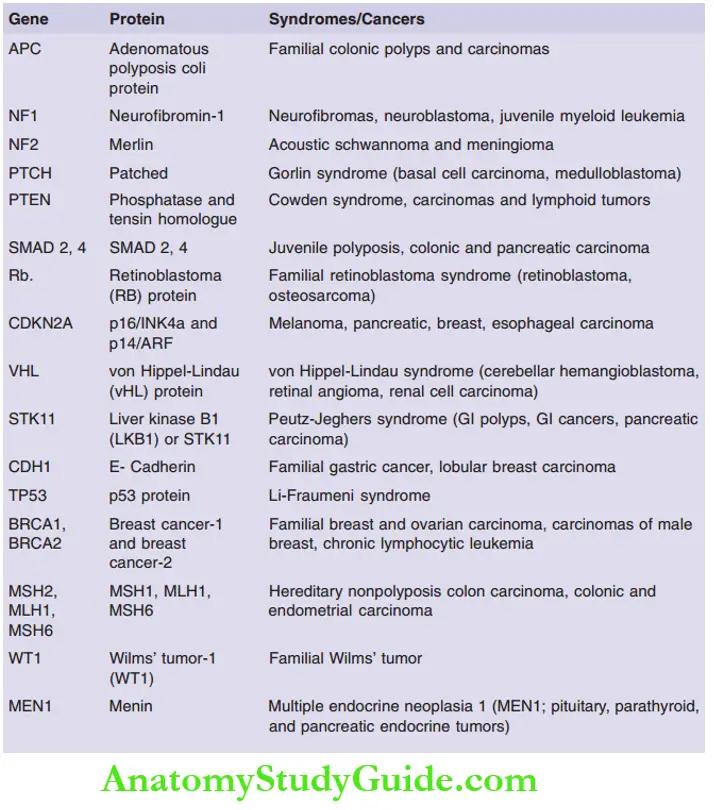
Question 9. Discuss tumor suppressor genes with examples. Describe in detail about any
two with their mechanisms and functions.
Answer:
Tumor suppressor genes
- Definition: It is a protein or gene that is associated with the suppression of any of the hallmarks of the cancer
- Apply brakes to cell proliferation
- Abnormalities in these genes lead to cell proliferation
Examples of tumor suppressor genes and their associations
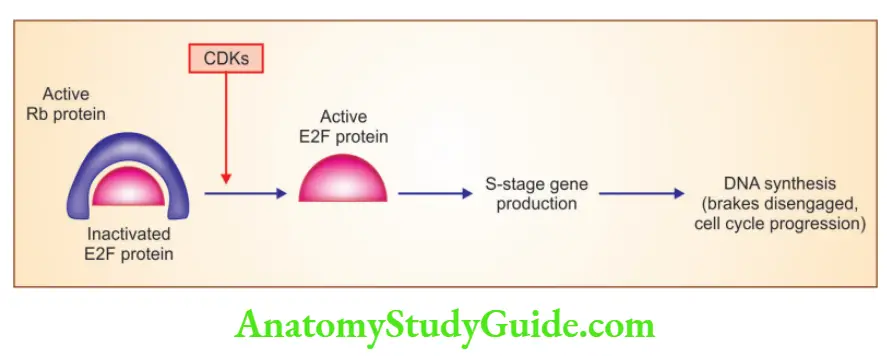
1. RB: Governor of proliferation
- Exists in an active hypophosphorylated state in quiescent cells and an inactive hyperphosphorylated state in cells passing through the G1/S cell cycle transition
- RB phosphorylation is inhibited by cyclin-dependent kinase inhibitors (p16/ INK4a)
- Hypophosphorylated RB is in complex with E2F transcription factors and inhibits the cell to go into the S phase
- RB is phosphorylated by the cyclin D-CDK4, cyclin D-CDK6, and cyclin E-CDK2 complexes, releasing E2F, resulting in the activation of the S phase of the cell cycle
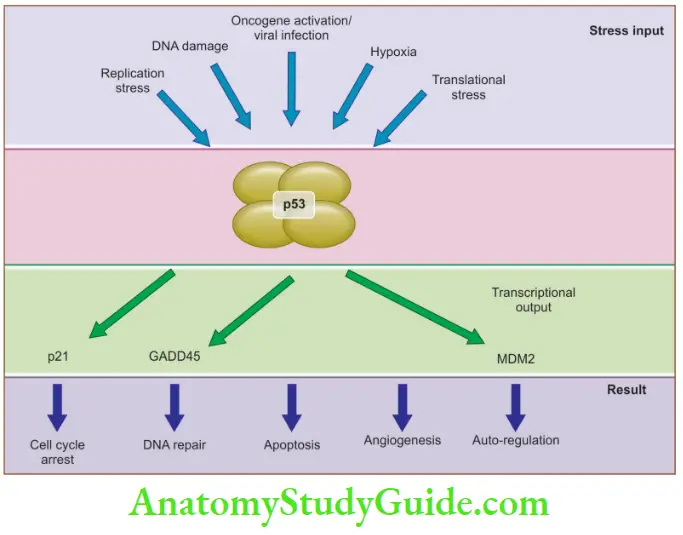
2. TP53: Guardian of the genome
- Regulates cell cycle progression, DNA repair, cellular senescence, and apoptosis
- Located on chromosome 17p13.1
- Most frequently mutated gene in human cancers
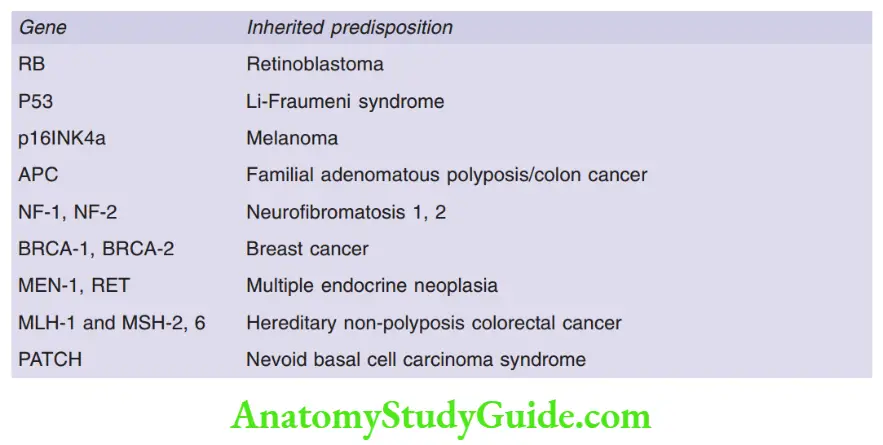
- Li-Fraumeni syndrome: Individuals who inherit one mutated TP53 allele, are at increased risk of developing sarcomas, breast cancer, leukemias, brain tumors, and adrenal cortical carcinomas
- Levels of p53 are negatively regulated by MDM2
- HPV-E6 proteins bind p53 and promote its degradation
p53 inhibits neoplastic transformation by:
- Activation of temporary cell cycle arrest (quiescence), induction of permanent cell cycle arrest (senescence), or triggering of programmed cell death (apoptosis)
p53 brings cell cycle arrest by activation of:
- p21, GADD45 (growth arrest and DNA damage), and BAX gene
Question 10. Write a short note on APC- β-catenin-WNT signaling pathway. Mention the role of E-cadherin in this pathway.
Answer:
APC: Gatekeeper of Colonic Neoplasia
- APC (5q21) gene mutation is associated with familial adenomatous polyposis
- APC and β-catenin are components of the WNT signaling pathway
In the absence of WNT, APC, and β-catenin in colonic epithelial cells form a macromolecular complex, resulting in the destruction of β-catenin - When colonic epithelial cells are stimulated by WNT molecules, β-catenin degradation does not occur and the later translocates to the nucleus and binds to TCF (transcription cell factor) resulting in cell cycle progression
- P Also, when APC is mutated, as in colonic polyps and cancers, β-catenin translocates to the nucleus, resulting in cell cycle progression
- β-catenin, which acts as a proto-oncogene, is mutated in hepatoblastoma and hepatocellular carcinomas
E-Cadherin
- β-catenin binds to the cytoplasmic tail of E-cadherin, a cell surface protein, that maintains intercellular adhesiveness
- Germline loss of function mutations of the E-cadherin gene, known as CDH1, is seen in familial gastric carcinoma
- Reduced E-cadherin expression is noted in the esophagus, colon, breast, ovary, and prostate cancers

Question 11. Write a short note on the neurofibromatosis gene.
Answer:
Classified into two types
1. Neurofibromatosis 1 (NF-1)
Encodes neurofibromin, which is a GAP (GTPase activating protein), that acts as a brake on RAS signaling
Predisposes to neurofibromatosis type 1, in which patients can develop numerous benign neurofibroma and optic nerve gliomas
2. Neurofibromatosis 2 (NF-2)
- Encodes neurofibromin 2 or merlin
- Germline mutations in the NF2 gene predispose to neurofibromatosis type 2
- Individuals with a mutation in the NF2 gene predisposes to benign bilateral schwannomas of the acoustic nerve, sporadic meningiomas, and ependymomas
Question 12. Write a short note on the Warburg effect.
Answer:
The Warburg effect
- Cancer cells demonstrate high levels of glucose uptake
- Cancer cells prefer aerobic glycolysis as a preferred source of energy and not oxidative phosphorylation
- Even though oxidative phosphorylation generates 36 molecules of ATP per molecule of glucose in contrast to only 2 molecules of ATP per molecule of glucose by aerobic glycolysis
- Aerobic glycolysis provides rapidly dividing tumor cells with metabolic intermediates that are needed for the synthesis of cellular components, whereas mitochondrial oxidative phosphorylation does not
Implication
- “Glucose-hunger” of tumor cells is used to visualize the tumors via positron emission tomography (PET) scanning
- In PET scanning, patients are injected with 18F-fluorodeoxyglucose, a nonmetabolizable derivative of glucose that is preferentially taken up into tumor cells
Question 13. Discuss in brief autophagy.
Answer:
Autophagy
- Severe nutrient deficiency, in which the cells arrest their growth, and cannibalize their own organelles, proteins, and membranes as carbon sources for energy production
- Tumor cells may use autophagy to become “dormant,” which allows cells to survive hard times for long periods
- These tumor cells are resistant to therapies that kill actively dividing cells and thus result in therapeutic failure
Question 14. Write a short note on familial cancer syndromes.
Answer:
Autosomal dominant inherited cancer syndromes
Defects in DNA repair systems like mismatch repair, nucleotide excision repair, and recombination repair—predispose to carcinomas
Examples:
1. Hereditary nonpolyposis colon cancer syndrome:
- Occurs due to defects in DNA mismatch repair genes
- Autosomal dominant disorder, associated with familial carcinomas of the colon affecting predominantly the cecum and proximal colon
- Microsatellite instability: Hallmark of patients with DNA-mismatch repair genes
- Germline mutations in MSH2 and MLH1 genes are most commonly implicated
2. Xeroderma pigmentosum:
Patients have a defect in the nucleotide excision repair pathway
Increased risk for the development of cancers of the skin exposed to UV light
Question 15. Classify chemical carcinogens and discuss in brief the steps involved in chemical carcinogenesis.
Answer:
Major chemical carcinogens
1. Direct-acting carcinogens:
- Alkylating agents
- Anticancer drugs (cyclophosphamide, chlorambucil, nitrosoureas, and others) Acylating agents
- Dimethylcarbamyl chloride
2. Procarcinogens that require metabolic activation:
- Polycyclic aromatic hydrocarbons: Benzo [a] pyrene
- Aromatic amines: Benzidine, dimethylamino azobenzene
- Microbial products: Aflatoxin B1, griseofulvin
- Others: Nitrosamine and amides, vinyl chloride, nickel, chromium, insecticides, fungicides
Steps involved in chemical carcinogenesis
1. Stage of initiation:
- All initiating chemical carcinogens are highly reactive electrophiles (electron-deficient atoms) that can react with nucleophilic (electron-rich) sites in the cell, DNA
- Initiation leads to nonlethal damage to the DNA that cannot be repaired
2. Stage of promotion/ progression during cancer development:
- Mutated cell passes on the DNA lesions to their daughter cells
- Resulting in proliferation induced by the promoter, with additional mutations and the formation of a malignant tumor
Question 16. Write a short note on radiation injury.
Answer:
Radiation energy in the form of UV rays of sunlight or ionizing radiation is carcinogenic
Ultraviolet rays
- UV rays can be divided into three wavelength ranges: UVA (320–400 nm), UVB (280–320 nm), and UVC (200–280 nm)
- UVB is responsible for the induction of cutaneous cancers, for example, squamous cell carcinoma, basal cell carcinoma, and melanoma of the skin
- UVB carcinogenicity is due to the formation of pyrimidine dimers in DNA
- UVC, a potent mutagen, is filtered out by the ozone layer surrounding the earth
Ionizing radiation
- Electromagnetic (X-rays, λ rays) and particulate (α particles, β particles, protons, neutrons) radiations are all carcinogenic
- Predisposes to myeloid leukemias, thyroid, breast, lung, and salivary glands carcinomas
Question 17. Mention oncogenic viruses. Discuss in detail oncogenic DNA viruses.
Answer:
Microbial carcinogenesis
- Oncogenic RNA viruses: Human T-cell leukemia virus type 1
- Oncogenic DNA Viruses: Human papillomavirus, Epstein-Barr virus (EBV), hepatitis B virus (HBV), Merkel cell polyomavirus, and Kaposi sarcoma herpes virus (HHV-8)
1. Human papillomavirus:
- High-risk HPVs (types 16 and 18): Implicated in squamous cell carcinomas of the cervix, anogenital region, and head and neck (tonsillar mucosa)
- HPV-6 and HPV-11 (low-risk HPVs): Responsible for genital warts
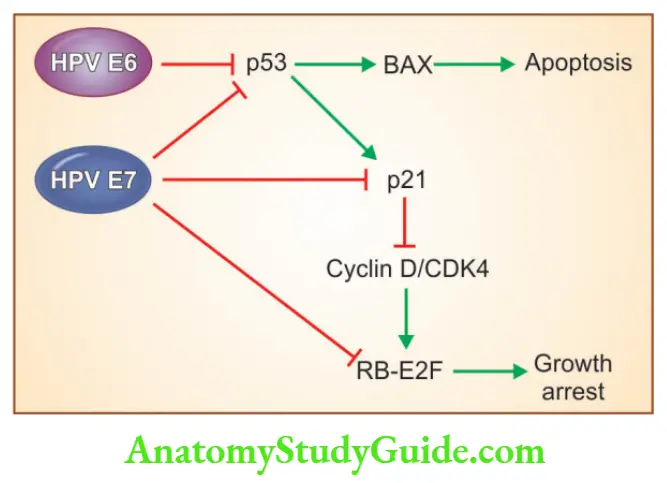
- The oncogenic potential of HPV is related to products of two viral genes: E6 and E7 HPV E6 protein
- This leads to the degradation of p53
- Stimulates the expression of TERT, the catalytic subunit of telomerase HPV E7 protein
- Binds to the RB protein and displaces the E2F transcription factors that are normally sequestered by RB, promoting progression through the cycle
- Inactivates the CDK inhibitors p21 and p27
2. Epstein-Barr virus is implicated in the pathogenesis of:
- Burkitt lymphoma, B-cell lymphomas in immunosuppressed individuals, Hodgkin lymphoma, nasopharyngeal carcinomas, T-cell lymphoma, and natural killer (NK) cell lymphoma
Points to remember:
- EBV uses the complement receptor CD21 to attach to and infect B cells
- EBV also possesses an oncogene, i.e. latent membrane protein-1 (LMP-1)
- LMP-1 activates NF-B and JAK/STAT signaling pathways and promotes B-cell survival and proliferation
- In Burkitt lymphoma, EBV leads to the acquisition of t(8;14), resulting in increased c-myc expression and resultant B-cell proliferation
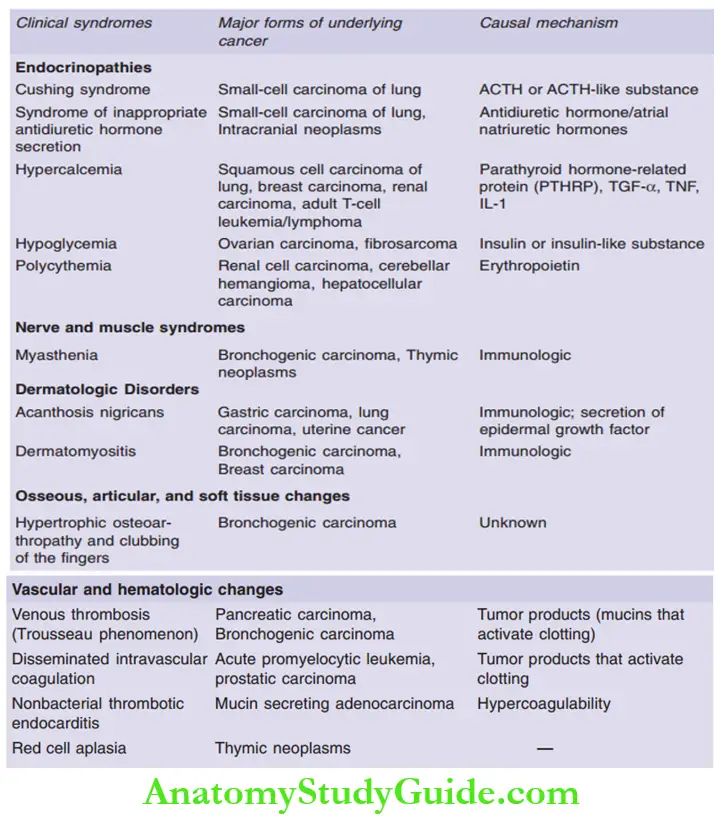
Question 18. Write a note on Paraneoplastic syndromes.
Answer:
Question 19. Write a short note on Grading and staging of tumors.
Answer:
Grade of a tumor
- Based on the degree of differentiation of the tumor cells
- Can be well-differentiated, moderately differentiated, or poorly differentiated
- Poorly differentiated cancers show more aggressive behavior
Stage of a tumor
- Is based on the size of the primary lesion, its extent of spread to the regional lymph nodes, and the presence or absence of blood-borne metastases
- American Joint Committee on Cancer Staging (AJCC) is the major staging system currently in use
- AJCC includes classification based on the TNM system—T for the primary tumor, N for regional lymph node involvement, and M for metastases
Question 20. Write a short note on laboratory diagnosis of cancer.
Answer:
Lab diagnosis of cancer can be characterized into several subcategories
1. Histological and cytological methods
Depends on the several sample approaches:
1. Excision or biopsy:
- Appropriate site of biopsy, tissue perseveration in formalin for processing, and special investigations such as cytogenetics, flow cytometry, and molecular diagnostics have to be taken care of
2. Fine needle aspiration:
- The procedure involves aspirating cells and fluid with a small-bore needle, followed by cytological examination of the stained smear
- Most commonly used for palpable lesions in the breast, thyroid, and lymph nodes
3. Cytologic smears:
- Methods to screen carcinoma cervix patients
- Also useful in endometrial carcinoma, lung carcinoma, bladder, and prostatic tumors, gastric carcinomas, and for the identification of tumor cells in abdominal, pleural, joint, and cerebrospinal fluids
2. Immunohistochemistry is useful in:
- Categorization of undifferentiated malignant tumors
- Determination of site of origin of metastatic tumors
- Detection of molecules that have prognostic or therapeutic significance, for example, estrogen receptor and progesterone receptor positivity in breast cancers
3. Flow cytometry: useful in B- and T-cell lymphomas and leukemias
4. Molecular and cytogenetic diagnostics:
- Diagnosis of malignant neoplasms
- Prognosis of malignant neoplasms
- Detection of minimal residual disease
- Diagnosis of hereditary predisposition to cancer
Question 21. Enumerate major tumor markers with examples.
Answer:
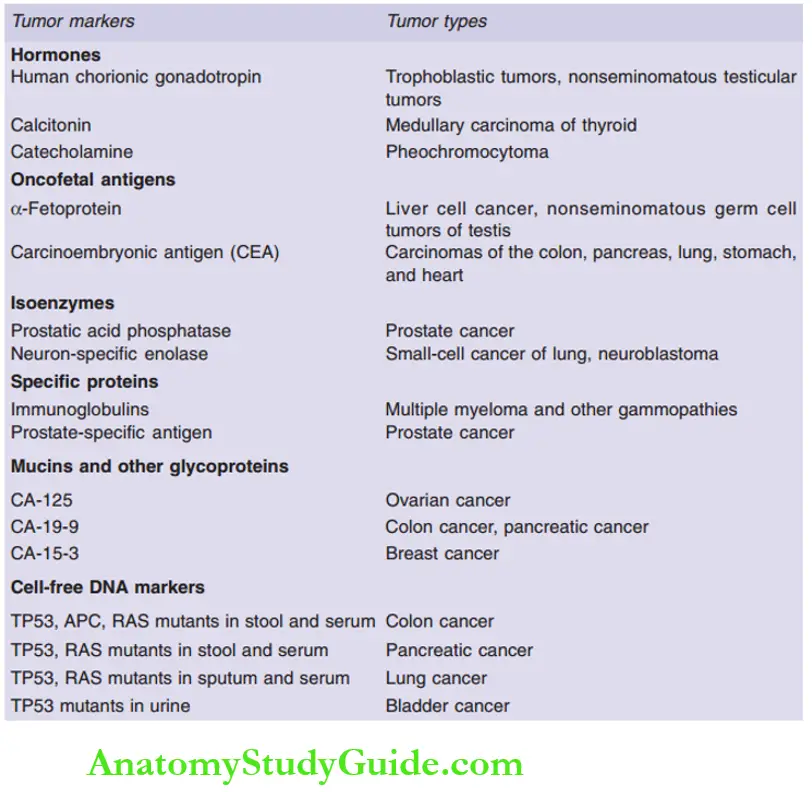
Leave a Reply