Plasmodium Species And Babesia
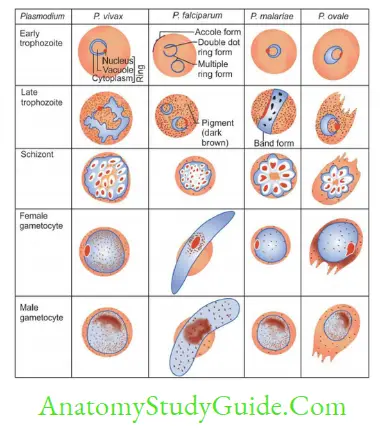
Plasmodium
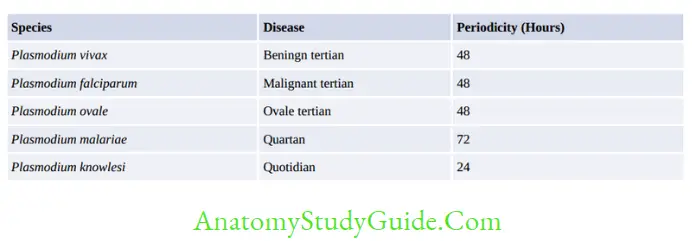
Plasmodium spp. causing malaria as follows:
Table of Contents
Read And Learn More: Micro Biology And Immunology Notes
Life Cycle
- Definitive host: Female Anopheles mosquitoes
- Intermediate host: Man
- Infective form to man: Sporozoites present in the salivary gland of mosquito.
- Infective form to man when transmitted by blood transfusion or vertical mode- trophozoite
- When transmitted by blood transfusion or vertical mode the difference in the life cycle is:
- There is no liver stage, and the infective form is trophozoite> merozoite
- Hypnozoites are not produced
- Hence, there is no relapse
- So no need of primaquine
- Infective form to mosquito- Gametocyte
- To infect mosquitoes- the gametocytes should be mature, viable, and count > 12 per cubic mm of blood.
- Asexual cycle in man:
- After the mosquito bite: Sporozoites are discharged to blood, carried to liver
- Liver cycle (pre or exoerythrocytic cycle): Sporozoites transform to trophozoites → Pre-erythrocytic schizont undergoes schizogony to produce → PE merozoites
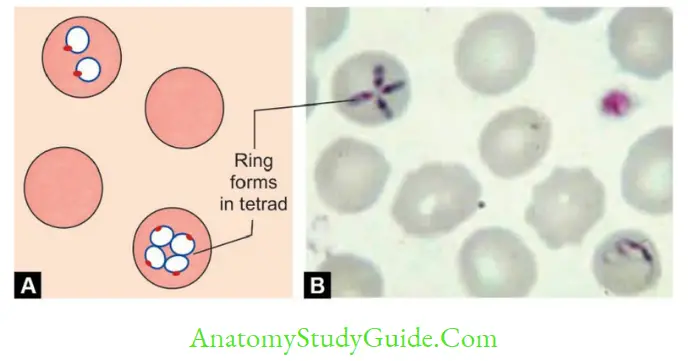
- RBC cycle: PE merozoites infect RBCs → transform to early trophozoites (ring form)→ late trophozoites → erythrocytic schizont → Undergoes schizogony to produce merozoites
- The release of merozoites leads to the appearance of clinical manifestations
- Merozoites either attack RBCs to repeat the cycle or transform to gametocytes which infect mosquito
- Sexual cycle (Sporogony): Begins when gametocytes infect the female anopheles mosquito and then transform to gametes → zygote → ookinete → oocyst → sporozoites.
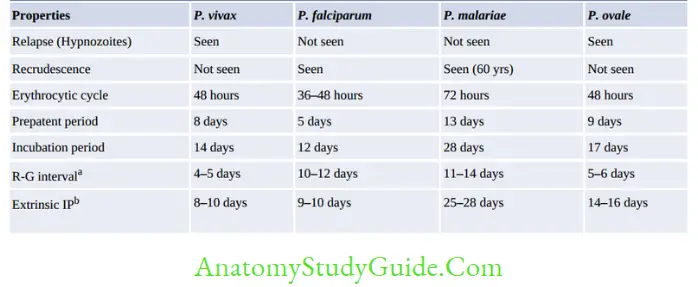
Recrudescence
- Seen in P. falciparum and P. malariae infections
- Falciparum malaria: Recrudescence is due to the persistence of drug-resistant parasites and fever reappears after 2–3 weeks of completion of treatment.
- In P. malariae infection, long-term recrudescences are seen for as long as for 60 years.
This is due to the long-term survival of erythrocytic stages at a low undetectable level in blood.
Relapse
- Due to hypnozoites (resting stage) which may reactivate after 2-3 years of malaria
- Seen in P. vivax and P. ovale
- Treatment of relapse: Primaquine
Pathogenesis of P. falciparum
- Sequestration, i.e. holding back the parasite in the blood vessels of deep visceral organs like the brain, kidney, etc. leads to vascular occlusion.
- Cytoadherence (binding of RBC to endothelium) – Pf EMP (P. falciparum erythrocyte membrane protein-1)
- Rossetting- unparasitized RBC clump together with parasitized RBC (become more spherical and rigid, and are less filterable than uninfected cells)
- Cytokines released: d/t GPI-glycosyl phosphatidyl Inositol
- Antigenic diversity of Pf EMP.
Immunity
- Nature of Hemoglobin:
- Sickle cell anemia trait: Protective from P. falciparum
- Thalassemia trait: Protective from P. falciparum
- Fetal Hb: Protective from P. falciparum
- Nature of RBC:
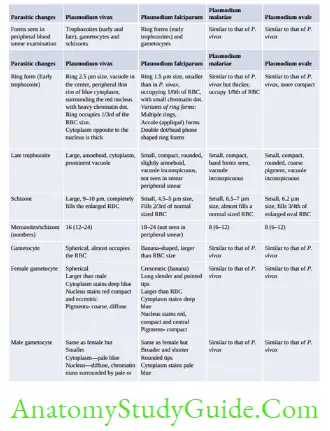
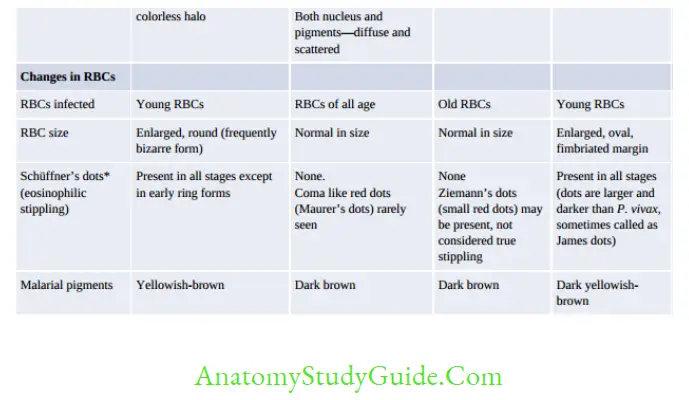
- P. falciparum: Infect All RBC,
- P. malariae: Infect old RBC,
- P. ovale/vivax: Infect Young RBC
- Nature of Enzyme- G6PD deficiency: Protective from P. falciparum
- Ovalocytosis: RBC is rigid, so protected from P. falciparum
- Duffy negative RBC: Protected from P. vivax
- HLA-Bw53 and haplotypes bearing DRW13.02 antigen and R111 gene
- Nutritional status: Has a paradoxical effect
Epidemiology
Situation in World
- WHO region: The tropical zone is affected the most. According to WHO Malaria Report 2017, the WHO African Region (90%) was affected the worst, followed by the South-East Asia Region (7%) and Eastern Mediterranean Region (2%)
- Incidence rate: It is about 63 cases per 1000 population at risk (incidence decreased by 18%, as compared to 2010). The WHO South-East Asia Region recorded the largest decline (48%)
- Most common species: In 2016, P. falciparum is the most common species worldwide; accounting for 99.7% of malaria cases in sub-Saharan Africa, Southeast Asia (66%),
Eastern Mediterranean regions (58%) and Western Pacific region (77%). P. vivax is the predominant parasite only in the American region (64%).
The situation in India (According to the NVBDCP Report 2016):
In 2016, 1.09 million malaria cases were reported in India with 331 deaths. The mortality rate declined by 70% compared to 2001
- P. falciparum was the predominant species; accounting for 65.53% of total cases followed by
P. vivax (34%) - Odisha accounted for the maximum malaria cases (41%); followed by Chhattisgarh (13.5%) and Jharkhand (13%). In Odisha, 85% of cases were due to P. falciparum; accounted for
23% of total deaths in India - P. malariae infections are <1% and reported from Karnataka (Tumkur and Hassan districts,largest foci), Chhattisgarh (Bastar area), Odisha, West Bengal, Madhya Pradesh, Tamil
Nadu, Kerala, and Assam - P. ovale is mainly confined to tropical Africa. Only a few cases are reported from India such as
- Odisha (Koraput district, 1st case of India), Chhattisgarh (Bastar area), Delhi, Assam,Gujarat and Kolkata.
Malaria Elimination in India
Malaria control in India has been operated through the NVBDCP since 2006.
- WHO has initiated The Global Technical Strategy for Malaria (2016–2030), which aims at the elimination of malaria by the year 2030.
Till date, 32 countries have already achieved the elimination status - In line with WHO, NVBDCP India has launched the National Framework for Malaria
Elimination (NFME) in 2016 with the vision of malaria elimination by 2030. In 2015, all the districts of India were stratified into four categories based on annual parasite incidence (API). - Category 0 Districts are historically considered to be without local transmission and have reported no case for the last 3 years.
The aim is to maintain malaria-free status in category zero districts or in districts where malaria transmission has been interrupted and prevent the re-introduction of malaria by strengthening surveillance. - Category 1 and 2 districts (i.e. districts having API <2)
- Category 3 districts (having API ≥2) will be brought into the pre-elimination and elimination phase by 2022.
Clinical Features of Malaria
- Fever, Sweating
- Anemia
- Splenomegaly (enlarged spleen)
- Irritability
- Coma, Retinal Hemorrhages
- Algid Malaria (a shock-like syndrome)
- Respiratory distress syndrome.
Complications
- Cerebral malaria
- Black water fever
- Tropical splenomegaly syndrome
- Malarial hyperpyrexia
- Bleeding/disseminated intravascular coagulation
- Hypoglycemia
- Renal failure
- Severe normochromic, normocytic anemia
- Tropical splenomegaly syndrome
- Gastrointestinal disorders.
- Algid malaria
- Nephrotic syndrome: P. malariae infection
- Promotes Burkitt’s Lymphoma.
Plasmodium knowlesi
- It is a malaria parasite of monkeys but can also affect humans, recently reported from Asia.
- Maximum cases have been reported from Malaysia (highest), Thailand, and Myanmar followed by a few reports from Indonesia, Vietnam, the Philippines, and Singapore.
The largest foci is located at Malaysian Borneo; 3,122 cases have been reported between 2004–2015. - The only report from India has documented from Andamans, where 11% of archived samples showed positive of P. knowlesi 18SrRNA gene.
- Blood smear examination: Early trophozoite- resembles to P. falciparum and Late trophozoite to P. malariae.
- Produces an acute illness and relatively high parasitemia compared to that of P. malariae.
- Paroxysms of fever occur daily (quotidian malaria) because of the short RBC cycle.
- Treatment: It responds well to chloroquine or primaquine. As the disease rapidly progresses, treatment should be promptly started.
Laboratory Diagnosis
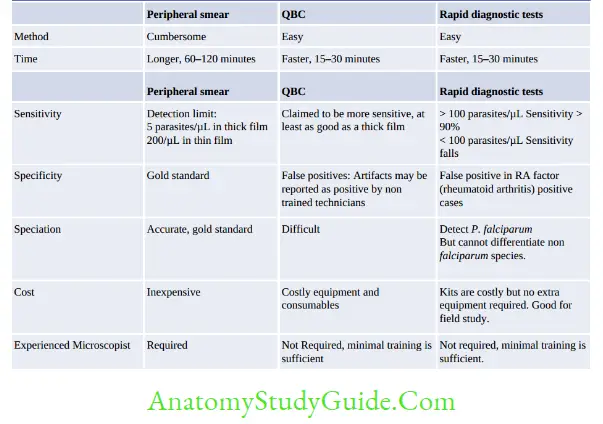
Microscopic Tests
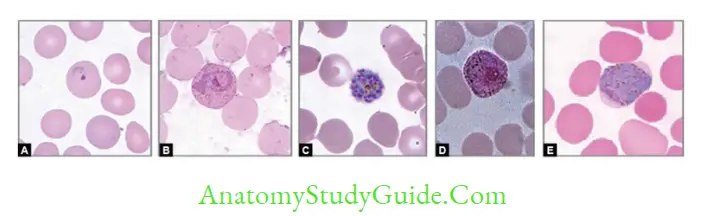
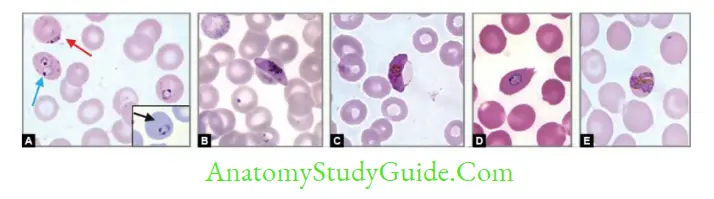
- Examination of peripheral blood smears (thin and thick blood films): Gold standard method:
- Thick smear: More sensitive (40 times), used for quantification and malaria pigment detection
- Thin smears: Used for speciation
- The feathery tail end of the smear should be examined
- At least 200–300 oil immersion fields should be examined before being considered as negative
- Stains used:
- JSB (Jaswant Singh and Bhattacharya) stain – Used in malaria control program in India
- Leishman’s, Giemsa, Field’s, Wright’s
- Fluorescence microscopy (Kawamoto technique)- by Acridine orange stain
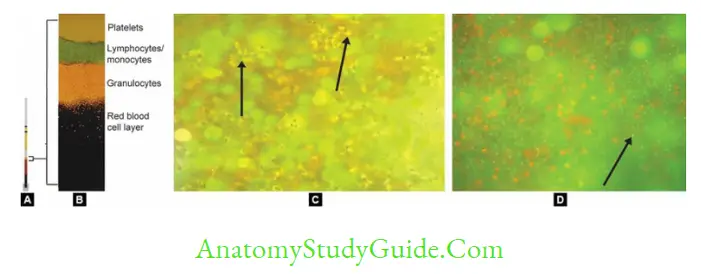
- Quantitative Buffy coat examination (QBC) Rapid method for detection of parasites:
- Blood is collected in a capillary tube coated with acridine orange + centrifuge the capillary tube + Examine the buffy coat region (junction of RBC & WBC) under UV.
Non-microscopic Tests
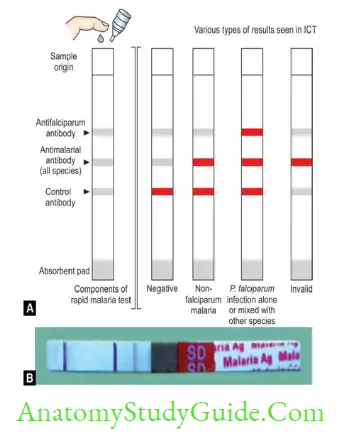
- Antigen detection tests: Rapid diagnostic tests (RDTs) or Immunochromatographic tests (ICTs)
- Rapid and simple but less sensitive, costly and may give false +ve in RA factor +ve cases
- pLDH and Aldolase: Common to all Plasmodium species
- HRP-2 Ag detection: Specific for P. falciparum.
- Antibody detection methods:
- Epidemiological survey in malaria.
- Screening of blood bank: To identify the infected donors
- Culture of malarial parasites: Tragger and Jensen method using RPMI 1640 medium is used for research purposes
- Molecular methods: Options available are: (1) Nested multiplex PCR (targeting 18S rDNA; detection limit of 10 to 100 copies/μL), (2) Real-time PCR (quantification and speciation
of parasites) and (3) LAMP assay- It is 100 times more sensitive than that of thick blood smear.
- Speciation can be done.
- Drug-resistance genes can be detected.
Treatment
Uncomplicated Benign Malaria in India
- Chloroquine is still the drug of choice for uncomplicated benign malaria in India. It is given 25 mg/kg divided over three days.
- Relapse rate of vivax malaria is around 30% in India. Primaquine is given as 0.25 mg/kg daily for 14 days to prevent relapse.
Complicated or Falciparum Malaria in India
- Treatment of falciparum malaria in India is based on area resistant or sensitive to chloroquine.


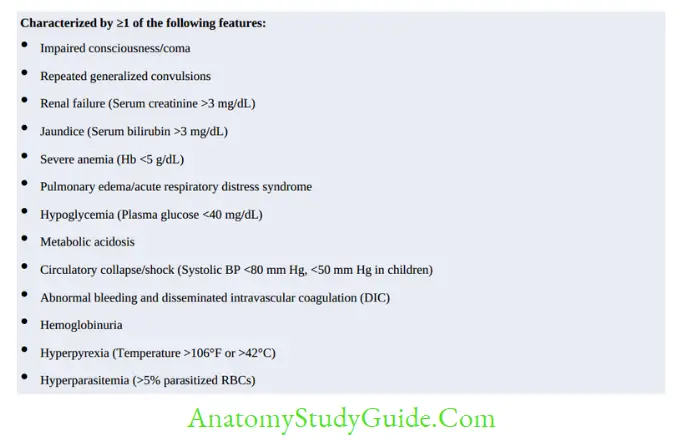

Note:
In pregnancy, quinine is preferred in the first trimester.
In severe P. vivax malaria, treatment is the same as of P. falciparum, except primaquine is given for 14 days.
For artemisinin derivatives, switch over to oral therapy when the patient becomes stable or after 24 hours of parenteral therapy whichever is later.
Antimalarial Drug Resistance
Drug Resistance in Falciparum Malaria
- World’s Highest Burden: Border areas of Thailand, Cambodia, and Myanmar possess the highest risk for resistance to chloroquine and other classes such quinine, sulfadoxine-pyrimethamine, halofantrine, and mefloquine.
- Many of the strains of P. falciparum are multiple-drug resistant (MDR), which is defined as resistance to at least ≥3 classes of antimalarial drugs
- Artemisinin resistance has not be reported from the World.
- India: Chloroquine resistance in P. falciparum has been reported since 1973 (first case from Assam).
- The highest chloroquine resistance in India has been reported from northeast states
- Currently, 117 districts are considered highly endemic for chloroquine resistance in P.falciparum (≥10% treatment failure); which include 67 districts of 7 North-east states and 50 districts from Andhra Pradesh, Chhattisgarh, Jharkhand, Madhya Pradesh, and Odisha.
- The important factors that contribute to the emergence of resistance are:
- Longer half-life of drug
- Mutation of the parasite for resistance
- Inadequate and irregular usage of drug
- Host immunity.
- Combination of drugs: The development of resistance can be delayed by a combination of drugs, i.e. combining one drug that rapidly reduces parasite biomass with a partner drug that can remove any residual parasites, e.g. ACT.
Drug Resistance in Vivax Malaria
Only sporadic cases of resistance to chloroquine and/or primaquine in some areas have been reported such as India, Myanmar, Indonesia, Papua, New Guinea, Brazil, Guyana, Colombia and Solomon Islands.
Mechanism of Drug Resistance
- Chloroquine resistance in P. falciparum: Occurs due to mutations in the genes encoding the transporter proteins such as PfCRT (P. falciparum chloroquine transporter) and PfMDR1 (P. falciparum multidrug resistance gene 1).
- Mutation in the PfMDR1 gene leads to resistance to other antimalarials like amodiaquine, mefloquine, and halofantrine.
- Resistance to antifolates such as sulfadoxine, pyrimethamine, and proguanil is due to point mutation in DHFR (dihydrofolate reductase) gene.
- Resistance to artemisinins has not be reported yet. Monotherapy with artemisinins is banned in India as it promotes resistance.
WHO Guideline for Assessing Degree of Resistance
- Early treatment failure (ETF): Development of danger sign or severe malaria (fever >37.5°C) on day 0–3 and parasitemia on day 3 is 25% higher than day 0 count.
- Late clinical failure (LCF): Development of danger sign or severe malaria (fever >37.5°C) in the presence of parasitemia from day 4 to day 28, without a previous meeting of any criteria of ETF.
- Late parasitological failure (LPF): Presence of parasitemia on any day from 7 days to 28 days and fever less than 37.5°C; without previous meeting of any criteria of early and late treatment failure.
- Adequate clinical and parasitological response (ACR): Absence of parasitemia on day 28 irrespective of fever, without previous meeting of any criteria of early and late treatment failure.
Prophylaxis Against Malaria
Prophylaxis against malaria includes chemoprophylaxis, vector control strategies and vaccine prophylaxis.
Chemoprophylaxis
Chemoprophylaxis is recommended for travelers, migrant laborers and military personnel exposed to malaria in highly endemic areas.
- For short-term chemoprophylaxis (<6 weeks): Doxycycline is recommended, should be started 2 days before travel and continued for 4 weeks after leaving the malaria endemic area.
- Long-term chemoprophylaxis (>6 weeks): Mefloquine is recommended and administered two weeks before, during, and four weeks after leaving the area.
Vector Control Strategies
- Anti-adult measures
- Residual spraying of houses with insecticides such as dichlorodiphenyltrichloroethane (DDT), malathion, and fenitrothion is highly effective against adult mosquitoes. Space application of pesticide in the form of fog or mist by ultra-low volume method of
pesticide dispersion - Individual protection: by using insecticide-treated bed nets, repellents, and protective clothing.
- Residual spraying of houses with insecticides such as dichlorodiphenyltrichloroethane (DDT), malathion, and fenitrothion is highly effective against adult mosquitoes. Space application of pesticide in the form of fog or mist by ultra-low volume method of
- Anti-larval measures
- Larvicide: Use of mineral oil or Paris green has been extensively used to kill mosquito larvae and pupae
- Source reduction (to reduce the mosquito breeding sites): Includes environmental sanitation, water management and improvement of the drainage system
Biological larvicide: Gambusia affinis (fish) and Bacillus thuringiensis (bacteria) can be used to kill the mosquito larva.
Vaccination for Malaria
Till date, there is no vaccine licensed for human use. RTS, S/AS01 This is the most successful malaria trial (completed phase III); started in 2005 in Africa (Kenya and Ghana) by GlaxoSmithKline (GSK).
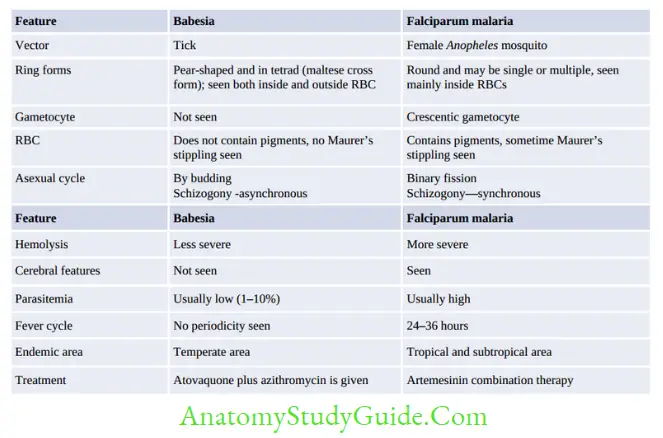
Babesia
- Intraerythrocytic protozoa
- Produces Malaria illness in animals and Zoonotic causes opportunistic infection to human
- Not found in India
- Vector: Tick-borne
- Treatment: Clindamycin with oral quinine atovaquone and azithromycin.
Leave a Reply