Spread Of Tumours
One of the cardinal features of malignant tumours is its ability to invade and destroy adjoining tissues (local invasion or direct spread) and disseminate to distant sites (metastasis or distant spread). About 90% of cancer-related deaths occur due to metastatic disease.
Table of Contents
Read And Learn More: General Pathology Notes
Local Invasion (Direct Spread)
Benign Tumours:
Most benign tumours form encapsulated or circumscribed masses that expand and push aside the surrounding normal tissues without actually invading, infiltrating or metastasising.
Malignant Tumours:
Malignant tumours also enlarge by expansion and some well-differentiated malignant tumours may even be partially encapsulated, for example, Follicular carcinoma thyroid.
- But characteristically, cancers are distinguished from benign tumours by invasion, infiltration and destruction of the surrounding tissue, besides spreading to distant sites or metastasis (described below).
- In general, tumours invade via the route of least resistance, though eventually, most cancers recognise no anatomic boundaries.
- Often, cancers extend through tissue spaces, permeate lymphatics, blood vessels, and perineural spaces and may penetrate a bone by growing through nutrient foramina.
- More commonly, the tumours invade thin-walled capillaries and veins rather than thick-walled arteries. Dense compact collagen, elastic tissue and cartilage are some of the tissues which are sufficiently resistant to invasion by tumours.
- The mechanism of direct invasion of malignant tumours is discussed along with that of metastasis below.
Read And Learn More: General Pathology Notes
Metastasis (Distant Spread)
Metastasis (meta = transformation, stasis = residence) is defined as the spread of the tumour by invasion in such a way that discontinuous secondary tumour mass/masses are formed at the site of lodgement.
- Besides anaplasia, invasiveness and metastasis are the two other most important features to distinguish malignant from benign tumours.
- Benign tumours do not metastasise while all the malignant tumours can metastasise, barring a few exceptions like gliomas of the central nervous system, basal cell carcinoma of the skin and dermatofibrosarcoma protuberans.
- Generally, larger, more aggressive and rapidly-growing tumours are more likely to metastasise but there are some exceptions.
- About one-third of malignant tumours at presentation have evident metastatic deposits while another 20% have occult metastasis.
Routes of Metastasis:
Cancers may spread to distant sites by following pathways:
- Lymphatic spread
- Haematogenous spread
- Spread along body cavities and natural passages (trans coelomic spread, spread along epithelium-lined surfaces, spread via cerebrospinal fluid, implantation).


Lymphatic and vascular routes of metastasis are more common, together called lymphovascular (LV) spread.
1. Lymphatic Spread:
In general, carcinomas metastasise more commonly by lymphatic route while sarcomas favour the haematogenous route. However, some sarcomas may also be spread by lymphatic pathways. The involvement of lymph nodes by malignant cells may be of two forms:
- Lymphatic permeation: The walls of lymphatics are readily invaded by cancer cells and may form a continuous growth in the lymphatic channels called lymphatic permeation.
- Lymphatic emboli Alternatively: The malignant cells may detach to form tumour emboli to be carried along the lymph to the next draining lymph node.
- The tumour emboli enter the lymph node at its convex surface and are lodged in the subcapsular sinus where they start growing. Later in the course of disease progression, the whole lymph node may be replaced and enlarged by the metastatic tumour.

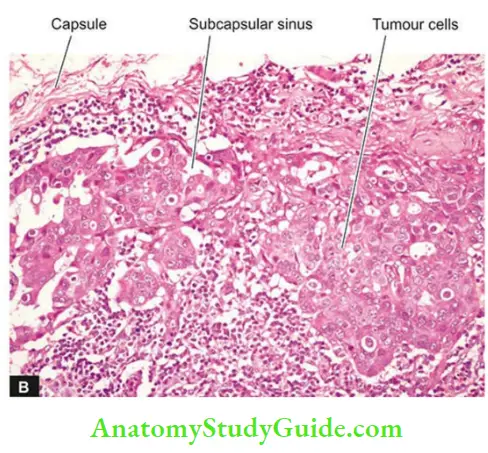
A few characteristics of the lymphatic spread of malignant tumours are as follows:
- Generally, regional lymph nodes draining the tumour are invariably involved in producing regional nodal metastasis.
- For example: frocarcinoma breast to axillary lymph nodes, From cancer of the thyroid to lateral cervical lymph nodes, Bronchogenic carcinoma to hilar and Para-tracheal lymph nodes etc.
- However, all regional nodal enlargements are not due to nodal metastasis because necrotic products of tumour and antigens may also incite regional lymphadenitis of sinus histiocytosis type.
- Sometimes, lymphatic metastases do not develop first in the lymph node nearest to the tumour because of venous-lymphatic anastomoses or due to obliteration of lymphatics by inflammation or radiation, so-called skip metastasis.
- Other times, due to obstruction of the lymphatics by tumour cells, the lymph flow is disturbed and tumour cells spread against the flow of lymph causing retrograde metastases at unusual sites.
- For example: Metastasis of carcinoma prostate to the supraclavicular lymph nodes and metastatic deposits from Bronchogenic carcinoma to the axillary lymph nodes.
- Virchow’s lymph node is nodal metastasis to the supraclavicular lymph node from cancers of abdominal organs, for example, Cancers of the stomach, colon, and gallbladder.
Lymph nodes in the vicinity of the tumour perform multiple roles—as initial barrier filters, and in the destruction of tumour cells, while later providing fertile soil for the growth of tumour cells. The mechanism of the lymphatic route of metastasis is discussed later under the biology of invasion and metastasis.
2. Haematogenous Spread:
Blood-borne metastasis is the more common route for sarcomas but certain carcinomas also frequently metastasise by this mode, especially those of the lung, breast, thyroid, kidney, liver, prostate and ovary.
- The sites where blood-borne metastasis commonly occurs are The Liver, Lungs, Brain, Bones, Kidney and Adrenals, All of which provide ‘good soil’ for the growth of ‘good seeds’, i.e. seed-soil theory postulated by Ewing and Paget a century ago.
- However, a few organs such as the spleen, heart, and skeletal muscle generally do not allow tumour metastasis to grow.
- The spleen is an unfavourable site due to the open sinusoidal pattern which does not permit tumour cells to stay there long enough to produce metastasis.
- In general, only a proportion of cancer cells are capable of clonal proliferation in the proper environment; others die without establishing metastasis.
A few features of haematogenous metastasis are as under:
- Systemic veins drain blood into vena cavae from limbs, head neck and pelvis. Therefore, cancers of these sites more often metastasise to the lungs.
- Portal veins drain blood from the bowel, spleen and pancreas into the liver. Thus, tumours of these organs frequently have secondaries in the liver.
- Pulmonary veins provide another route for the spread of not only primary lung cancer but also metastatic growth in the lungs.
- Blood in the pulmonary veins carrying cancer cells from the lungs reaches the left side of the heart and then into systemic circulation and, thus, may form secondary masses elsewhere in the body.
- Arterial spread of tumours is less likely because they are thick-walled and contain elastic tissue which is resistant to invasion.
- Nevertheless, arterial spread may occur when tumour cells pass through the pulmonary capillary bed or through pulmonary arterial branches which have thin walls.
- Cancers of the kidneys, adrenals, bones, limbs and uterus, which are drained by systemic veins, spread to the lungs via the pulmonary artery.
- Retrograde spread by blood route may occur at unusual sites due to retrograde spread after venous obstruction, just as with lymphatic metastases.
- Important examples are vertebral metastases in cancers of the thyroid and prostate.
Grossly: Blood-borne metastases in an organ appear as multiple, rounded nodules of varying size, scattered throughout the organ. Sometimes, the metastasis may grow bigger than the primary tumour.
- At times, metastatic deposits may come to attention first without an evident primary tumour.
- In such cases searching for a primary tumour may be rewarding, but sometimes the primary tumour may remain undetected or occult. Metastatic deposits, just like primary tumours, may cause further dissemination via lymphatics and blood vessels
Microscopically: The secondary deposits generally reproduce the structure of the primary tumour. However, the same primary tumour on metastasis at different sites may show varying grades of differentiation, apparently due to the influence of the local environment surrounding the tumour for its growth.
3. Spread Along Body Cavities And Natural Passages:
Uncommon routes of spread of some cancers are by seeding across body cavities and natural passages as under:
- Transcoelomic spread: Certain cancers invade through the serosal wall of the coelomic cavity so that tumour fragments or clusters of tumour cells.
Break off and carried in the coelomic fluid and are implanted elsewhere in the body cavity. The peritoneal cavity is involved most often, but occasionally pleural and pericardial cavities are also affected. - A few examples of trans coelomic spread are as follows:
- Carcinoma of the stomach seeding to both ovaries (Krukenberg tumour).
- Carcinoma of the ovary spreads to the entire peritoneal cavity without infiltrating the underlying organs.
- Pseudomyxoma peritonei is the gelatinous coating of the peritoneum from mucin-secreting carcinoma of the ovary or appendix.
- Carcinoma of the bronchus and breast seeding to the pleura and pericardium.
- Spread along epithelium-lined surfaces: It is unusual for a malignant tumour to spread along the epithelium-lined surfaces because intact epithelium and mucus coat are quite resistant to penetration by tumour cells.
- However, exceptionally a malignant tumour may spread through:
- The fallopian tube from the endometrium to the ovaries or vice-versa



-
- Through the bronchus into the alveoli, and
- Through the ureters from the kidneys into the lower urinary tract.
- Spread via cerebrospinal fluid: Malignant tumours of the ependyma and leptomeninges may spread by the release of tumour fragments and tumour cells into the CSF and produce metastases at other sites in the central nervous system.
- Implantation: There are isolated and rare case reports of the spread of some cancers by implantation by surgeon’s scalpel, needles, sutures, and direct prolonged contact of cancer of the lower lip causing its implantation to the apposed upper lip.
Cell Biology Of Invasion And Distant Metastasis
The spread of cancer by invasion into surrounding tissues and to distant sites to form metastases or secondary masses are defining features of malignancy.
- It includes genetic alterations, epigenetic phenomena, and co-option of non-neoplastic stromal elements in cancer.
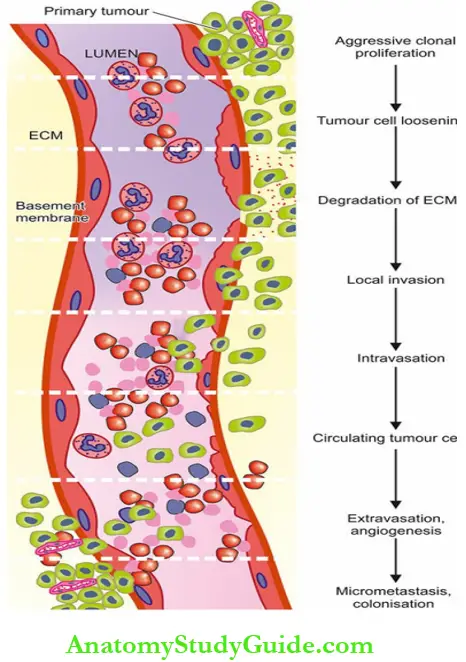
- The mechanism of cancer spread involving a series of complex events in cell biology called ‘invasion-metastasis cascade’ is illustrated and described below:
1. Aggressive clonal proliferation of primary tumour:
A primary cancer spreads by developing rapidly proliferating clones of cancer cells:
- More aggressive tumours develop intratumoral heterogeneity i.e. in the mass of monoclonal tumour cells, a subpopulation or clone of tumour cells.
- Starts proliferating aggressively, having the potential to spread locally or develop into metastasis.
- Certain metastatic oncogenes have been found involved in the process of metastasis.
- These metastatic oncogenes are SNAIL and TWIST genes which encode transcription factors that promote epithelial-to-mesenchymal transition (EMT) (see below).
- The Tumour microenvironment (TME) plays an important role in the spread of the tumour. It includes tumour angiogenesis, tumour stroma, and the extent and type of host immune response.
- In the growing tumour, newly-formed vessels are particularly more vulnerable to invasion because these evolving vessels are leaky and are in direct contact with cancer cells.
2. Tumour cell loosening:
Normally, epithelial cells maintain cell-to-cell and cell-to-ECM interactions by cell adhesion molecule (CAM), E-(epithelial) cadherin, which acts to keep the cells glued to each other and to ECM, as well as to transmit signals.
- In epithelial cancers, E-cadherin is inactivated and its functions is lost due to EMT mediated by metastatic oncogenes, Snail And Swift.
- This results in the detachment of cancer cells from each other and from the ECM.
- There is also a loss of integrins, the transmembrane receptors, further favouring invasion.
3. Degradation of ECM:
There is upregulation of degrading and proteolytic enzymes, matrix metalloproteinases (MMPs) in almost all human cancers while the concentration of tissue inhibitor of metalloproteinases (TIMP) is decreased.
- These enzymes favour the degradation of ECM locally and contribute to local invasion.
- MMPs are also involved in cell proliferation, survival, immune response and angiogenesis.
4. Loss of basal polarity:
Normally, epithelial cells are oriented to the basement membrane due to integrins having receptors for basement membrane proteins, mainly laminin and fibronectin.
In cancer, there is the degradation of these basement membrane proteins, loss of tumour cell-toECM adhesiveness and generation of factors that promote tumour cell migration.
5. Intravasation:
The tumour cells after degrading the ECM and basement membrane are ready to migrate into the lumen of capillaries, venules or lymphatics for which the following mechanisms play a role:
- Autocrine motility factor (AMF), is a cytokine derived from tumour cells which stimulates receptor-mediated motility of tumour cells.
- Cleavage products of matrix components which are formed following degradation of ECM have properties of tumour cell chemotaxis, growth promotion and angiogenesis in cancer.
6. Circulating tumour cells (CTC):
Once the cancer cells are in circulation, they are faced with harsh conditions due to immune attack by the host defences and shear forces from blood flow and, therefore, must adapt to survive. CTCs may develop the following adaptations
- Develop a coat from constituents of the circulating blood and form the thrombus.
- Metabolic rewiring to avoid oxidative stress. Normally millions of CTCs are released into circulation but only a very small proportion of malignant cells (less than 0.1%) in the bloodstream survive to develop into metastasis.
- CTCs can be isolated as ‘liquid biopsy’ for cancer screening and for metastatic relapse risk assessment.
7. Extravasation and organ predilection:
Tumour cells in circulation (capillaries, venules, lymphatics) may either mechanically block these vascular channels.
- May initiate a growth there (mechanical hypothesis), or they attach to vascular endothelium and extravasate to the extravascular space.
- However, unlike intravasation where newly-formed vascular channels are leaky, extravasation for the tumour cells is more difficult since they have to cross the barrier of tightly bound endothelial cells, basement membrane and layer of pericytes.
The following factors are involved in extravasation:
- Chemokines and chemokine receptors on tumour cells
- CD44 adhesion molecule
Some organs such as the bone marrow and liver have highly-permeable sinusoidal vessels and are, thus, favoured sites for the development of metastasis (seed-soil hypothesis).
8. Micrometastasis, dormancy and colonisation:
The extravasated malignant cells are in a new microenvironment foreign to the cells that challenge their survival.
- It has been hypothesised that a few stimulators secreted by the tumour cells and the bone marrow are involved in pre-metastatic preparation to harbour the cells which then develop into a micrometastasis.
- Alternatively, the surviving cancer cells may go into dormancy or hibernation at the secondary site such as the bone marrow.
- The dormant cancer cells eventually proceed to form clinically detectable metastasis by colonisation.
- Although the exact mechanism of how these dormant cells escape host defences to develop overt metastasis is not known it has been hypothesised that metastasis-initiating cells possess stem cell-like properties to challenge host barriers.
- Metastatic deposits may further metastasise to the same organ or to other sites by forming emboli.
Spread of Tumours:
Malignant tumours invade and destroy adjoining tissues (local invasion or direct spread) and disseminate to distant sites (metastasis or distant spread).
- Most cancers spread to distant sites commonly by lymphatic or haematogenous route; some cancers spread along body cavities (trans-coelomic spread) and via natural passages (for example, Along bronchus, fallopian tubes, ureters, CSF etc).
- Carcinomas metastasise more commonly by lymphatic route while sarcomas favour the haematogenous route.
- Common sites of lymphatic metastasis are the regional nodes, while blood-borne metastases are common in the liver, lungs, bones, brain, kidneys and adrenals.
The mechanism of direct invasion and distant metastasis involves a series of changes in cell biology called the invasion-metastasis cascade. These changes are:
- Aggressive clonal proliferation in the primary tumour
- Loss of cell-cell and cell-ECM interaction
- Degradation of ECM
- Loss of basal polarity
- Intravasation of cancer cells,
- Tumour cells in circulation
- Extravasation of tumour cells and organ predilection, and
- Micrometastasis Dormancy and colonisation to develop into clinically detectable metastasis.
Leave a Reply