Structure of Immune System and Immune Response
Central Lymphoid Organs
Table of Contents
Bone Marrow
The progenitor T- and B-cells originate in bone marrow. Further development of B-cells occurs in bone marrow itself; whereas the progenitor T-cells proliferate in thymus.
Thymus
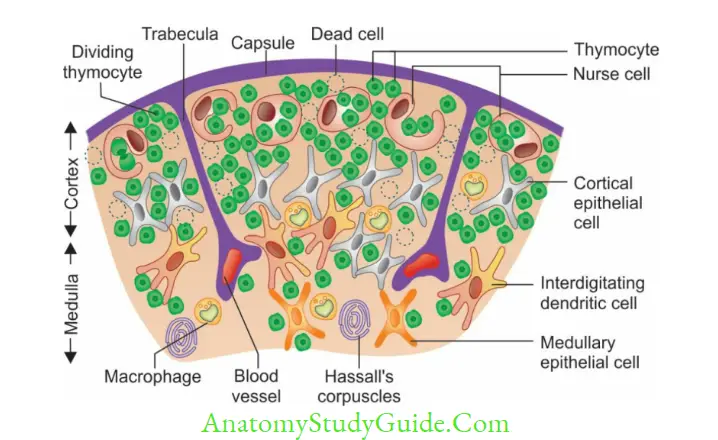
Thymus is developed in the embryonic life (third month) from third/fourth pharyngeal pouch. It is highly active at birth, reaches its peak size at puberty, and then it degenerates.
Structure: Thymus has two lobes, each is differentiated into an outer cortex and an inner medulla.
Read And Learn More: Micro Biology And Immunology Notes
- Cortex is densely populated and contains: Numerous thymocytes (thymic lymphocytes),epithelial cells and nurse cells (specialized epithelial cells with long membrane extensions that surround many thymocytes)
- Medulla is sparsely populated and contains: Few thymocytes, epithelial cells, dendritic cells and Hassall’s corpuscles (concentric layers of degenerating epithelial cells)
- Thymic hormones such as thymulin, thymopoietin and thymosin are produced which help in T-cell development.
- Maturation of T-cells:
- DN cell: T-cell precursors after entering into the thymus transform into double negative (DN) T-cells (CD4– CD8–)
- DP cells: Then the DN cells acquire CD4 and CD8 to become Double positive (DP) T-cells.
The DP cells have the following fates.- Death by neglect: Majority (95%) do not specifically recognize their MHC molecules and are destroyed
- Positive selection: The remaining 5% of DPT-cells undergo Positive selection
- Negative selection: Out of 5% of DPT-cells that are positively selected, 2–5% are selfreacting and hence they are negatively selected, i.e. destroyed. (Central tolerance)
- Mature T-cells: The remaining DP T-cells (2–5%) loose either CD4 or CD8 to form Mature
T-cells (e.g. CD4+ helper T-cells and CD8+ cytotoxic T-cells). They acquire thymus specific antigens and then are released into the circulation and migrate to the peripheral lymphoid organs where they respond to the antigenic stimulus.
Peripheral Lymphoid Organs
Lymph Node
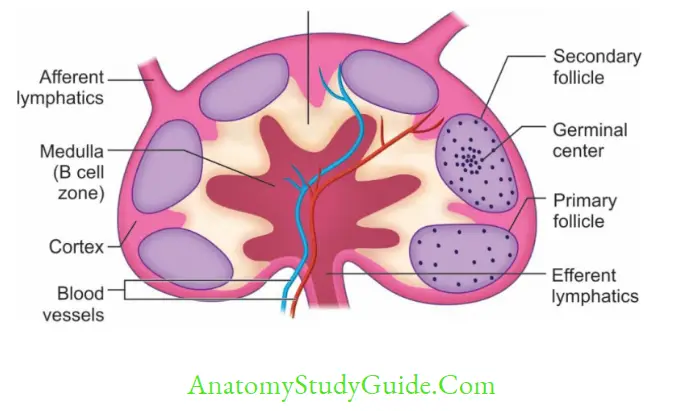
Lymph nodes are small bean-shaped organs; divided into three parts: cortex, medulla (both are B-cell areas) and paracortex (T-cell area).
Cortex contains lymphoid follicles which are mainly of two types.
- Primary lymphoid follicles: They are present before the antigenic stimulus; they are smaller and contain resting B-cells.
- Secondary lymphoid follicles: They are formed following contact with an antigen, contain activated B-cells (i.e. plasma cells and memory B-cells). They are divided into central area (germinal center) and peripheral zone (mantle area).
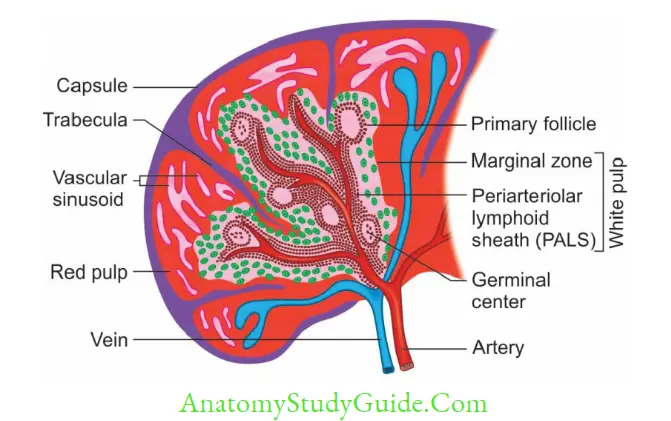
Spleen
Spleen is the largest secondary lymphoid organ. It is divided into two compartments; central white pulp and outer red pulp.
- White pulp has two parts: (1) Periarteriolar lymphoid sheath (T-cell area), (2) Marginal zone (B-cell area)
- Red pulp contains the sinusoids, filled with RBCs. The older and defective RBCs are destroyed here.
Defect in spleen: As spleen is the site of destruction of most of the microbes, abnormalities of spleen or splenectomy, often leads to an increased incidence of bacterial sepsis caused primarily by capsulated bacteria.
Mucosa Associated Lymphoid Tissue (MALT)
MALT are present lining the mucosa of intestine, respiratory, and urogenital tract.
MALT in intestine are the best studied MALT, present in different layers of intestinal wall:
- Submucosa contains Peyer’s patches, composed of lymphoid follicles
- Lamina propria contains loose clusters of lymphocytes and macrophages.
- Epithelial layer contains:
- Few specialized lymphocytes called intraepithelial lymphocytes (γδ T-cells)
- M cells: They are specialized flattened epithelial cells that do not have microvilli.
- They are lined by Secretory IgA which provide local or mucosal immunity.
Lymphocytes
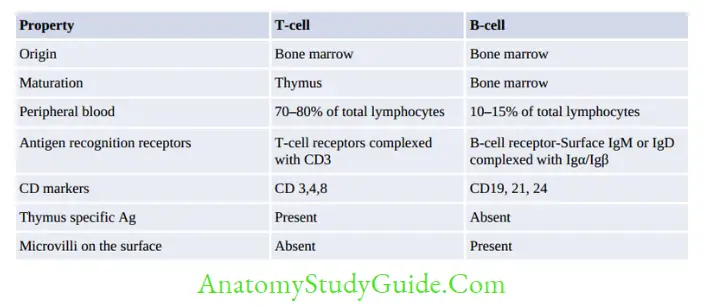
Lymphocytes can be of three types: T lymphocytes, B lymphocytes and NK (natural killer) cells.
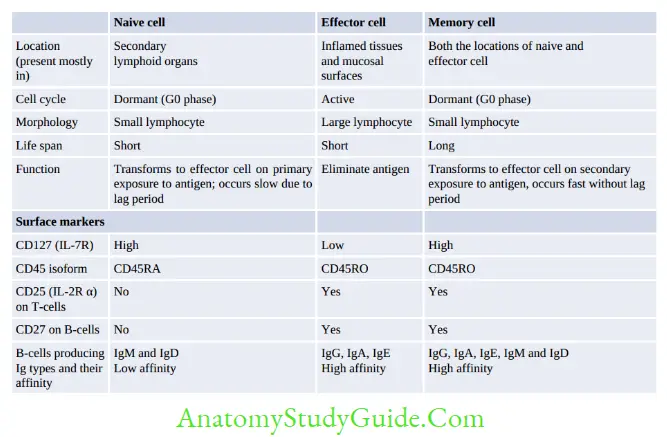
T- and B-cells can also be classified into naive lymphocytes (prior antigenic contact) and lymphoblasts (following antigenic contact) which eventually differentiate into effector cells or memory cells.
T Lymphocytes
There are two types of effector T-cells CD4+ helper T-cells and CD8+ cytotoxic T-cells. Rare subtypes are TREG cells and γδ T-cells
Regulatory T-cells (TREG cells, formerly known as suppressor T-cells):
- They provide tolerance to self-antigens (peripheral tolerance), and prevent the development of autoimmune disease.
- Surface markers: TREG cells possess surface markers such as CD4, CD25 and Foxp3
- A deficiency of Foxp3 receptors leads to a severe form of autoimmune disease known as Immune dysregulation, Polyendocrinopathy, Enteropathy X-linked (IPEX) syndrome.
γδ T-cells: They constitute 5% of total T-cells, express γ/δ chains of TCR chains; instead of α/β chains.
They lack both CD4 and CD8 molecules.
- They do not require antigen processing and MHC presentation of peptides.
- They are part of innate immunity as the γδ receptors exhibit limited diversity for the antigen.
- They are usually found in the gut mucosa, as intraepithelial lymphocytes (IELs).
- The function of γδ T-cells is not known, they may encounter the lipid antigens that enter through the intestinal mucosa.
B Lymphocytes
B-cells proliferate through various stages, first in bone marrow, then in peripheral lymphoid organs.
B-cell development in bone marrow is described below.
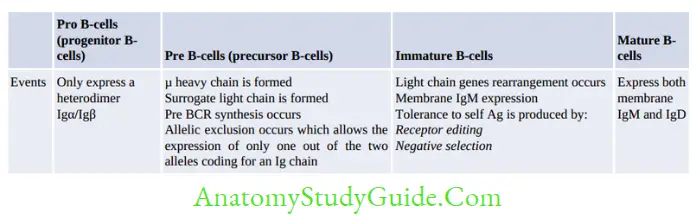
Development in peripheral lymphoid organs:
Immature B-cells migrate from bone marrow to peripheral lymphoid organs (lymph node and spleen) where they transform into mature or naive B-cells.
Following antigenic stimulus, the mature B-cells transform into activated B-cells (lymphoblasts) which further differentiate into either effector B-cells, i.e. plasma cells (majority) or memory B-cells.
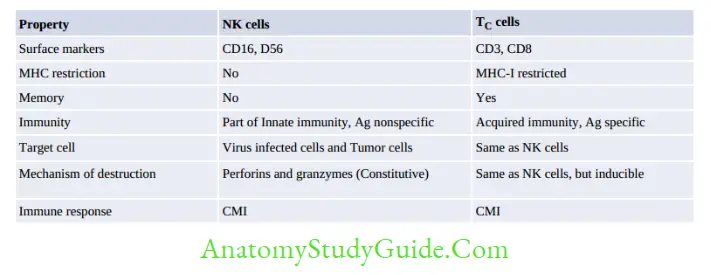
Natural Killer (NK) Cells
NK cells are large granular lymphocytes that constitute 10–15% of total lymphocytes, described later under CMI.
Other Cells of Immune System
Other cells of immune system include: Macrophages, dendritic cells, Granulocytes (e.g. neutrophils, eosinophil and basophils) and mast-cells.
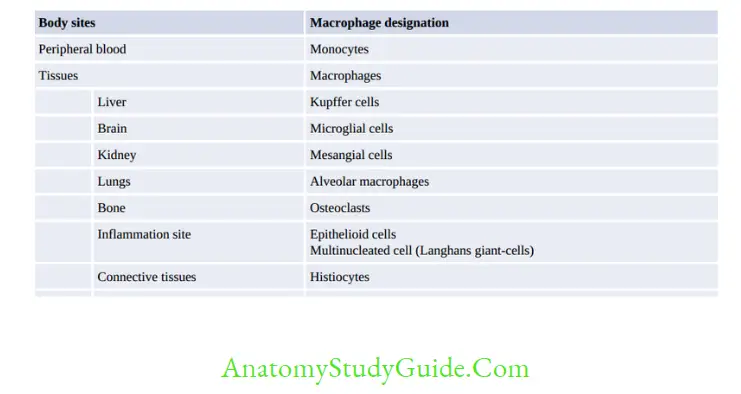
Macrophage
Monocytes/macrophages originate from bone marrow, from a separate granulocyte-monocyte progenitor cells. Monocytes are the largest blood cells present in blood. They do not divide and within 8 hours they migrate to tissues.
Two major functions of macrophage are Phagocytosis and Antigen presentation.
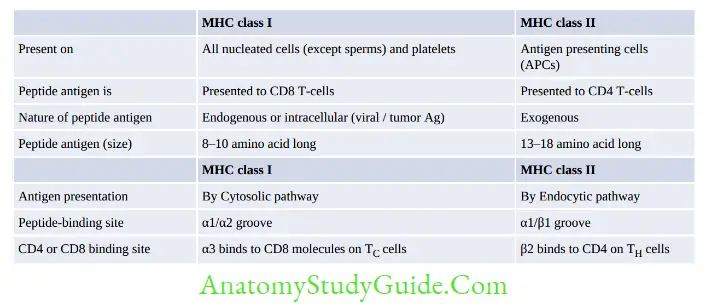
Major Histocompatibility Complex
- MHC molecules or human leukocyte antigens (HLA) serve as a unique identification marker for every individual as the genetic sequence of MHC genes is different for every individual.
- They also determine the histocompatibility between the donor graft and the recipient.
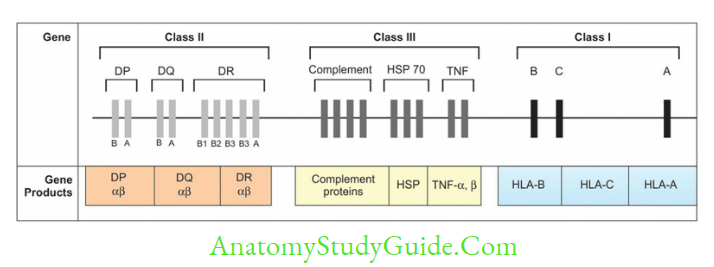
- In humans, HLA complex coding for MHC proteins are located in short arm of chromosome
- The genes are clustered in three regions named as MHC region-I, II and III
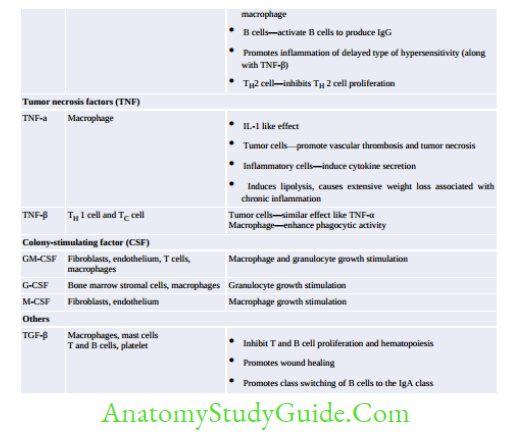
- MHC I and II help in antigen presentation to T-cells:
- MHC I presents intracellular antigen on viral/tumor cells to cytotoxic T-cells
- MHC II presents extracellular antigen on APCs to helper T-cells
- MHC III does not help in Ag presentation, but code for various proteins such as complement factors (C2, C4, C3 convertase, factor B and properdin), heat shock protein, TNF-α and β, and steroid 21-hydroxylases.
Immune Responses
Immune response refers to the highly coordinated reaction of the cells of immune system and their products. It has two arms Humoral or Antibody-Mediated Immune response (AMI) and Cell-Mediated Immune response (CMI)
- Bothe CMI and AMI interdependent, regulated by the helper T (TH) cells
- There is common pathway first before CMI/AMI; which involves antigen presentation to helper T-cells followed by activation of helper T-cells.
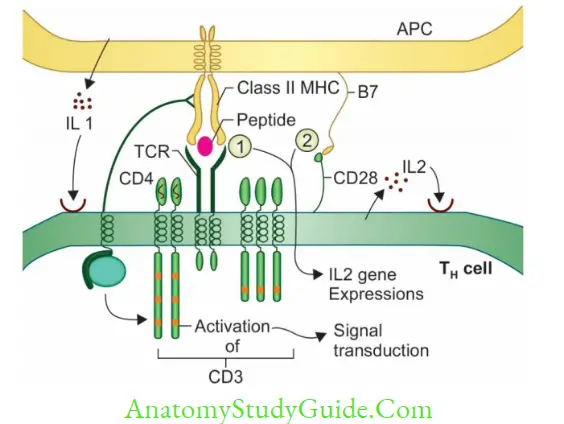
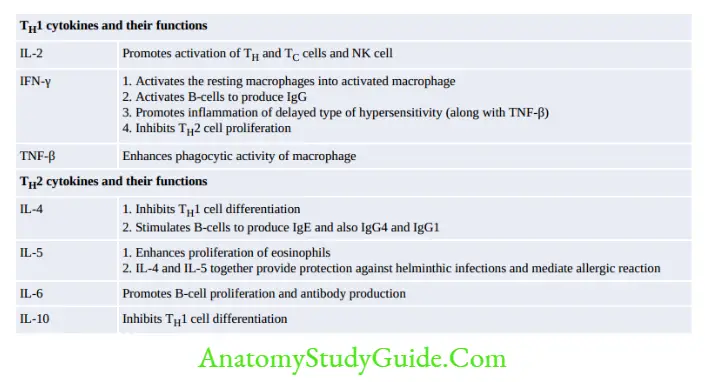
- Activated helper T-cells differentiate in to either TH1 or TH2 subsets. Induction of TH1 cells secrete cytokines that stimulate CMI whereas if TH2-derived cytokines induce the B-cells to produce antibodies.
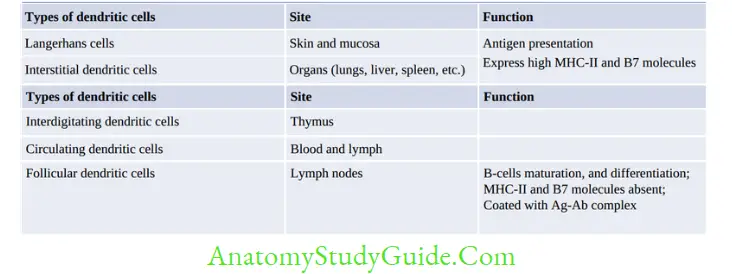
Antigen-Presenting Cells (APCs)
APCs implies to cells that present the antigenic peptide along with MHC class II to TH cells.
They may be grouped into:
- Professional APCs: e.g. Macrophages, Dendritic cells and B-cells
- Nonprofessional APCs: e.g. Fibroblasts (skin), Thymic epithelial cells, Pancreatic beta cells,
Vascular endothelial cells, Glial cells (brain) and Thyroid epithelial cells.
Helper T-cells (Activation and Differentiation)
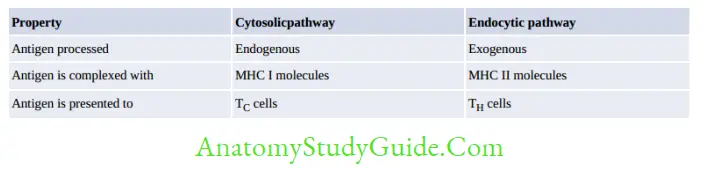
Activation of TH cells requires generation of these specific signals:
- Antigen-specific signal: Involves binding of antigenic peptide on APCs to TCR of TH cells.
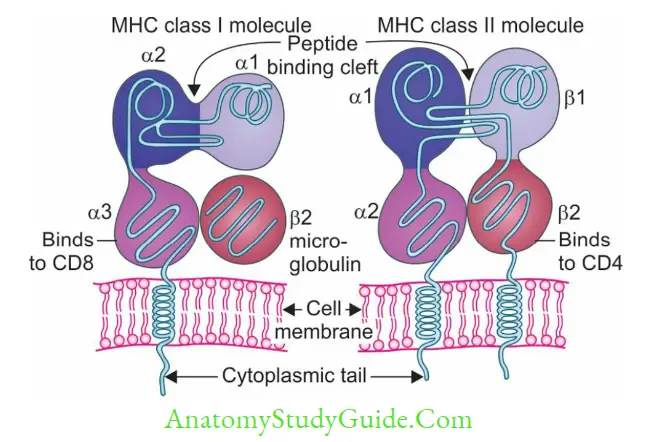
- CD4 molecules of TH cells also interact with β2 domain of MHC-II.
- Costimulatory signal involves binding of CD28 molecule on TH cells to B7 molecules on APCs.
- Cytokine signal: IL-1 secreted from APCs acts on TH cells.
Following signal transduction, naive TH cells differentiates into effector and memory T-cells.
Effector TH cells further differentiate into either TH1 or TH2 subsets, regulated by IL-12 which promotes TH1 subset proliferation.TH1 and TH2 derive cytokines mediates various functions.
Cell-Mediated Immune Response
CMI provides immunity against: (1) microbes residing in intracellular milieu (both obligate and facultative) (2) tumor cells, (3) Mediate delayed/type IV hypersensitivity (4) plays key role in transplantation immunity and graft-versus-host reaction.
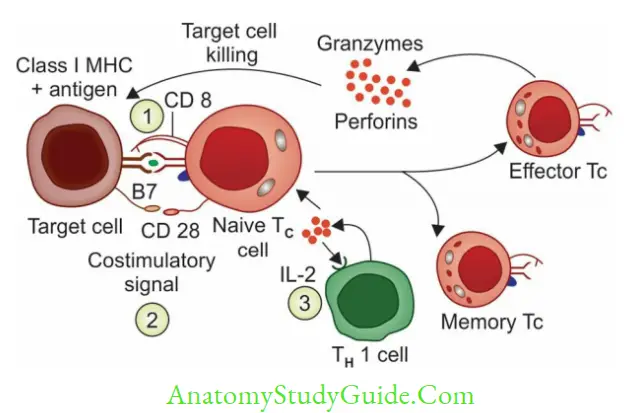
Cytotoxic T Lymphocytes
CD8TC cells are the principal effector cells of CMI. Activation of Naive TC cells requires these specific signals.
- Antigen-specific signal: TCR of naive TC cells binds to MHC I -peptide complex of target cells.
- CD8 of TC cells also interacts with a domain of MHC-I.
- Costimulatory signal: CD28 of naive TC cells interacts with B7 molecule on target cells.
- Cytokine signal: IL-2 (secreted by TH 1 cell) acts on TC cells
The activated T C cells produce two types of lethal enzymes; called (i) perforins (for pores on the target cells) and (ii) granzymes (destroy the target cells)
Natural Killer Cells
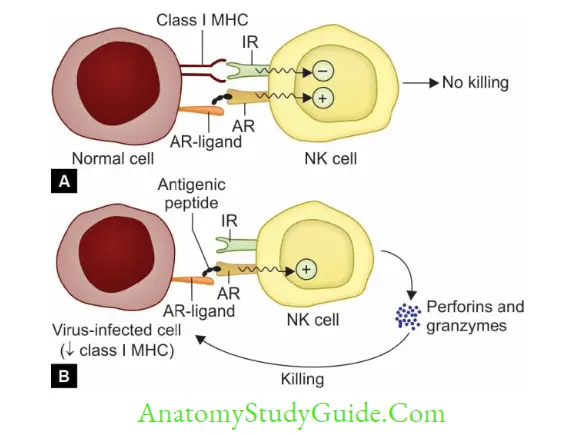
NK cells are large granular lymphocytes that constitute 10–15% of peripheral blood lymphocytes:
- They are derived from a separate lymphoid lineage. NK cells are cytotoxic but antigen nonspecific.
- They are part of innate immunity, act as first line of defense and do not require prior contact with the antigen.
- Mechanism of NK cell-mediated cytotoxicity:
- Receptor interaction: When activation receptors (e.g. NKR-P1, CD16) present on NK cells are engaged with ligands present on the target cells; NK cells become activated.
- Target cell destruction: is similar to that of TC cells, i.e. via secreting perforins and granzymes.
However, the NK cells enzymes are constitutively expressed (i.e. they are cytotoxic all the time, even without exposure to the antigen).
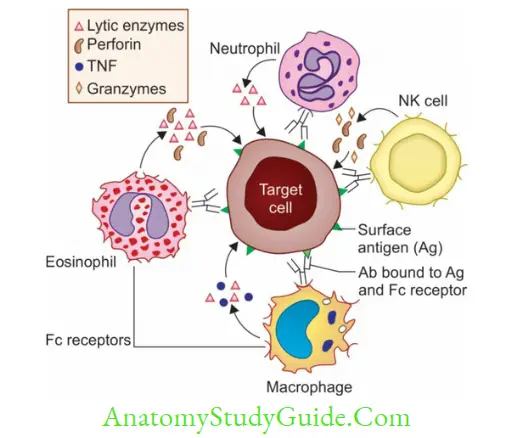
Antibody-Dependent Cell-Mediated Cytotoxicity (ADCC)
A number of nonspecific cytotoxic cells express receptors (FcR) on their surface that can bind to the Fc region of any Ig:
- These cells can bind to Fc portion of the antibody coated on the target cells, and subsequently cause lysis of the target cell by releasing various cytotoxic factors such as:
- NK cells secrete perforins and granzymes
- Neutrophils releases lytic enzymes
- Eosinophils can release lytic enzymes and perforins; protects against helminths
- Macrophages produce lytic enzymes and TNF
- Although these cytotoxic cells are nonspecific for the antigen, the specificity of the antibody directs them towards the specific target cells. This type of cytotoxicity is referred to as antibody-dependent cell-mediated cytotoxicity (ADCC).
Assessment/Detection of CMI
(1) Mixed-lymphocyte reaction (MLR), (2) Cell-mediated lympholysis (CML), (3) The graft versus host reaction (GVH) in experimental animals.
Humoral/Antibody Mediated ImmuneResponse (AMI)
AMI provides protection to the host by secreting antibodies; that prevent invasion of microbes present on the surface of the hosT-cells and in the extracellular environment but has no role against intracellular microbes.
AMI occurs through the following three sequential steps:
- Activation of B-cells carrying microbial antigen (B-cells act as APCs) and presenting to TH cells. This requires the following signals:
- Signal-1 is induced by the cross-linking of mIg on B cell membrane with the microbial antigen.
-
- Signal-2 provided by binding of CD40 on B-cell with CD 40L (ligand) on activated TH cells.
- Signal-3 Cytokines produced by the activated TH cells act on B-cells

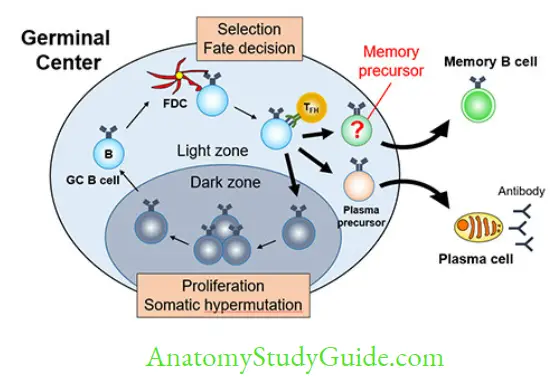

- Differentiation of B-cells into effector cells (plasma cells) and memory cells. This occurs in the germinal center of secondary lymphoid follicles.
- Effector functions: Production of secreted antibodies by plasma cells which in turn counteract with the microbes in many ways such as neutralization, opsonization, complement activation, ADCC, mucosal immunity, etc.
Leave a Reply