Acquired Immunodeficiency Syndrome (AIDS):
Since the initial recognition of AIDS in the United States in 1981, there have been tremendous advances in understanding of its epidemiology, aetiology, immunology, pathogenesis, clinical features and morphologic changes in various tissues and organs of the body.
Table of Contents
Although antiretroviral therapy is being widely used globally for such patients, research for finding a safe, cost-effective and efficient HIV vaccine has not yielded a definitive outcome, and thus worldwide efforts are directed at prevention by education, counselling and behavioural modifications.
Read And Learn More: General Pathology Notes
The definition and criteria for staging for AIDS are complex. Currently, US CDC classification system for HIV infection and AIDS is widely followed: stage 0, 1, 2, 3 and unknown.
These criteria rest on two parameters: specific clinical manifestations and decreased CD4+ T cell counts
Acquired Immunodeficiency Syndrome Epidemiology
AIDS is pandemic in distribution and is seen in all continents. As per UNAIDS Global Report 2017, an estimated 37 million people are living with AIDS globally which includes 50% women and 2 million children below 15 years of age.
About 1.8 million new cases are being added every year (about 5,000 new cases added per day). Region-wise, incidence remains highest in countries in the African continent; other countries in descending order of frequency are Middle-East, South and South-East Asia, Pacific countries, Latin America, Caribbean, East Europe, Central Asia,Western and Central Europe, and North America. Global efforts at HIV prevention and antiretroviral therapy treatment has lowered.
AIDS-related mortality by about 48% in 2016 (1 million deaths) in comparison with data in 2005 (1.9 million deaths), but it is still far from UN target of fewer than 500,000 death per year by 2020. The burden of AIDS in India is estimated at
2.1 million cases; four Southern states (Tamil Nadu, Andhra Pradesh, Telangana and Karnataka) comprise about 50% of all HIV positive cases (mostly contracted heterosexually), while NorthEast states account for 8% of all cases (more often among intravenous drug abusers).
Acquired Immunodeficiency Syndrome Etiologic Agent
AIDS is caused by an RNA (retrovirus) virus called human immunodeficiency virus (HIV).
There are 4 members of human retroviruses in 2 groups:
- Transforming viruses These are human T cell leukaemia-lymphoma virus (HTLV) I and II and are implicated in leukaemia and lymphoma (page 237).
- Cytopathic viruses This group includes HIV-1 and HIV-2, causing two forms of AIDS.
Most common cause of AIDS in the world is HIV-1, while HIV-2 is an etiologic agent for AIDS in numerous developing as well as developed countries.
Both HIV1 and HIV2 are zoonotic infections and their origin can be traced to chimpanzees and gorillas which are non-human primate reservoirs of HIV and the most likely source of original infection.
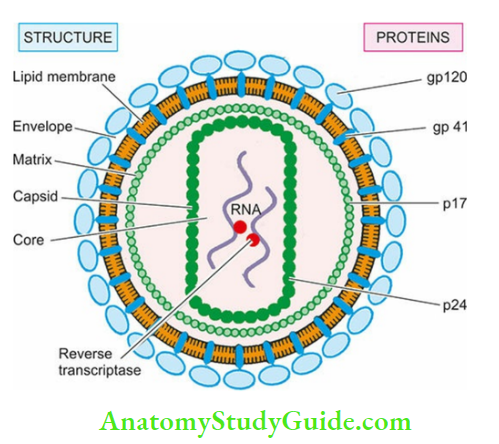
HIV-I virion or virus particle:
HIV-I virion or virus particle On electron microscopy is icosahedral structure, 100-140 nm in size. The virion buds from the surface of infected host cell and during this process it gets covered by a lipid bilayer derived from the host cell which incorporates host proteins into it.
- Cone-shaped core contains major capsid protein p24, matrix (or inner membrane) protein p17, nucleocapsid protein p7/9, two strands of viral genomic RNA and three viral enzymes (reverse transcriptase, protease, integrase). Capsid protein p24 is the most abundant and is used for detection of HIV infection.
- Host cell-derived outer lipid bilayer membrane of the virus is the envelope of virion which is studded with two viral glycoproteins at different locations: gp120 (externally) and gp41 (transmembranous). These proteins are significant for HIV infection of the cells.
Following genes code for the respective viral proteins:
- Gag (group antigen) for core proteins (capsid protein p24, matrix protein p17, nucleocapsid protein p7/9).
- Pol (polymerase) for reverse transcriptase, protease, integrase, and ribonuclease.
- Env (envelope) for the envelope proteins (gp120, gp41).
- Tat (transcription activator) gene (p14) for amplification of integrated viral DNA.
Based on molecular studies of viral genome of HIV, considerable heterogeneity has been reported, particularly in the region of envelop glycoproteins. Accordingly, there are subtypes of
HIV
- HIV-1 has further subtypes as M, N, O and P.
- HIV-2 has subtypes as A through K.
Acquired Immunodeficiency Syndrome Routes Of Transmission
Transmission of HIV infection occurs by one of the following routes and it varies in different populations:
1. Sexual transmission:
Sexual contact is the main mode of spread and constitutes 75% of all cases of HIV transmission.
Most cases of AIDS in the industrialised world occur in homosexual or bisexual males while heterosexual promiscuity seems to be the dominant mode of HIV infection in Africa and Asia.
Other sexually transmitted diseases (STDs) may act as cofactors for the spread of HIV, in particular gonorrhoea and chlamydial infection. Transmission from male-to male and male to female is more potent route than that from female to male.
2. Transmission via blood and blood products:
This mode of transmission is the next largest group (25%) and occurs in 3 types of high-risk populations:
- Intravenous drug abusers by sharing needles, syringes etc comprise a large group in the US.
- Haemophiliacs who have received large amounts of clotting factor concentrates from pooled blood components from multiple donors.
- Recipients of HIV-infected blood and blood products who have received multiple transfusions of whole blood or components like platelets and plasma.
3. Perinatal transmission:
HIV infection occurs from the infected mother to the newborn during pregnancy transplacentally, or in the immediate post-partum period through contamination with maternal blood, infected amniotic fluid or breast milk.
4. Occupational transmission:
Health care workers (HCW), laboratory workers and those engaged in disposal of waste of sharps are at higher risk of HIV infection by occupational exposure to HIV-infected material.
After a needle-stick injury, there is a small risk of seroconversion (~0.3%) and in all such cases, anti-retroviral therapy is started within 24-48 hours which reduces the risk markedly.
It is imperative that these workers follow the CDC guidelines for universal precautions which include disinfecting and sterilising all reusable devices and use of bleaching (hypochlorite) solution for disinfecting all blood spillage.
5. Transmission by other body fluids:
Although besides blood, HIV has been isolated and identified from a number of body fluids such as saliva, tears, sweat, urine, semen, vaginal secretions, cervical secretions, breast milk, CSF, synovial, pleural, peritoneal and pericardial fluid, there is no definite evidence that HIV transmission can occur by any of these fluids; isolated cases of such infection reported are in likelihood due to concomitant contamination with HIV-infected blood.
It may, however, be understood regarding spread of HIV infection that AIDS cannot be transmitted by casual non-sexual contact like shaking hands, hugging, sharing household facilities like beds, toilets, utensils etc.
It should also be appreciated that HIV-contaminated waste products can be sterilised and disinfected by most of the chemical germicides used in laboratories at a much lower concentration.
These are sodium hypochlorite (liquid chlorine bleach) (1-10% depending upon the amount of contamination with organic material such as blood, mucus), formaldehyde (5%), ethanol (70%), glutaraldehyde (2%), β-propiolactone. HIV is also heat-sensitive and can be inactivated at 56°C for 30 min.
Acquired Immunodeficiency Syndrome Pathogenesis
Pathogenesis of HIV infection is largely related to qualitative and quantitative deficiency of CD4+ T cells (helper T cells) resulting in profound suppression of cell-mediated immunity.
Besides immune system, HIV infection targets the central nervous system in particular. The course in the HIV disease from initial (primary) HIV infection to development of chronic advanced disease is complex and goes through different phases of the disease.
For the sake of understanding, pathogenic events in HIV disease are discussed under the following three headings:
- Events from primary HIV infection to established persistent infection
- Destruction of the immune system
- HIV infection of the central nervous system
1. Events from primary hiv infection to established persistent Infection:
Within hours after exposure to HIV infection, often from mucosal surfaces, the HIV virus enters the body and passes through following sequence.
- It crosses the lamina propria by using Langerhans cells or dendritic cells as transport.
- In the lamina propria, the virus seeks its primary target, CD4+ T cells, dispersed in the submucosa.
- The virus replicates in these lymphoid cells and is transported to draining lymphoreticular tissues (lymph nodes, GALT) where it establishes itself.
- GALT is particularly targeted by HIV which is rich in CD 4+ T cells and the virus depletes CD 4+ T memory cells there in particular.
- As the virus multiplies in lymphoid cells within days to weeks, it enters the bloodstream (viraemia) and the infection disseminates to the body.
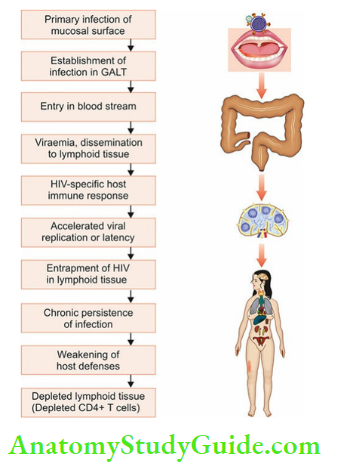
- Meanwhile, HIV-specific host immune response is elicited both by humoral (anti-HIV antibodies) and cell-mediated (HIV-specific cytotoxic T lymphocytes or CD 8+ T cells) immunity, which attempts to restrict viral replication.
- Eventually, the virus is trapped in the lymphoid tissues and establishes itself as a chronic persistent infection. In the process, it disrupts the architecture of lymph nodes.
- With the depletion of CD 4+ T cells, host immune defenses become weak while the surviving HIV-infected CD 4+ T cells continue to replicate virus rapidly and increase the viral burden.
2. Destruction Of the Immune System Eventually:
The immune system of the host is totally knocked off leaving behind a persistent reservoir of HIV-infected CD 4+ T cells. The pathogenic sequence involved in the life cycle of HIV infection in the target cells is schematically and is briefly outlined below:
Selective tropism for CD4 molecule receptor:
gp120 envelope glycoprotein of HIV has a selective tropism for cells containing CD4 molecules on their surface. These cells most importantly are CD4+ T cells (T helper cells); other such cells include monocyte-macrophages, microglial cells, epithelial cells of the cervix, Langerhans cells of the skin and follicular dendritic cells.
Initially, HIV on entering the body via mucosal surfaces by any route has tropism for macrophages (M-tropic) while later it becomes either dual tropic or T-tropic only and thus affects mainly CD4+ T cells which are the main target of attack by HIV.
Fusion and internalisation:
gp 120 of the virion combines with CD4 receptor on the target cell; binding and entry of the virion into the host cell is facilitated by cellular chemokine coreceptors for HIV, CCR-5 and CCR-4.
Dendritic cells too express C-type lectin receptors on their surface having a high affinity for gp120 envelope protein, facilitating the spread of virus to CD 4+ T cells.
Once gp envelope protein of HIV has combined with the CD4 molecule in association with the coreceptor (CCR 5 or 4), fusion and penetration into host cell membrane of the target cell occurs via the newly-exposed gp41 glycoprotein.
Uncoating and viral DNA formation:
Once the virion has entered the T cell cytoplasm, uncoating of the capsid protein is initiated followed by reverse transcription.
This forms a preintegration complex composed of viral RNA, enzymes, and associated proteins. As preintegration complex moves towards the nucleus of target host cell, viral reverse transcriptase causes reverse transcription of the genomic RNA into a single-stranded DNA.
Using the singlestranded DNA as a template, DNA polymerase copies it to make it double-stranded unintegrated linear DNA, while destroying the original RNA strands.
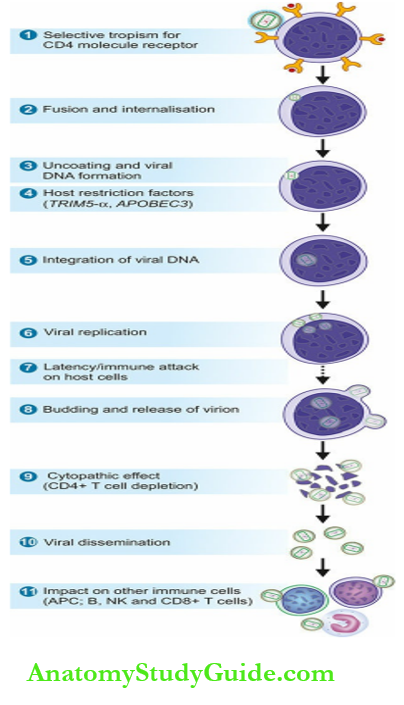
Host restriction factors:
The viral genome at preintegration complex may be restricted from further progression by two host cellular factors:
- Cytoplasmic TRIM5-α (TRIM= tripartite motif-containing protein 5-α): This is host restriction factor that interacts with capsid proteins of retroviruses in general, but fails to recognise HIV-1 capsid.
- APOBEC3 (apolipoprotein-B mRNA editing enzyme, catalytic polypeptide like 3) is a cellular protein which may bind to HIV virion in the cytoplasm before it enters the host nucleus but it fails to do so; instead, APOBEC3 is degraded by viral protein Vif (viral infectivity factor).
Integration of viral DNA:
The unintegrated viral DNA traverses from the cytoplasm of the host cell to its nucleus where it is integrated into the chromosomes by an enzyme, viral integrase.
Thus, the viral DNA integrates into the nuclear DNA of the host T cell. At this stage, viral particle is termed as HIV provirus.
Viral replication:
HIV provirus has become part of host cell DNA, and host cell DNA transcripts for viral RNA with the presence of tat gene.
Multiplication of viral particles is further facilitated by release of cytokines from T helper cells (CD4+ T cells): TH 1 cells elaborate IL-2 and IFN- γ, and TH2 cells elaborate IL-4, IL-5, IL-6, IL-10. RNA viral particles thus fill the cytoplasm of host T cell where they acquire protein coating. Released cytokines are also responsible for spread of infection to other body sites, in particular to CNS by TNF-α.
Latent period and immune attack:
In an inactive HIV-infected T cell, the infection may remain in latent phase for a long time, accounting for the long incubation period. Immune system attempts to act against the virus by the participation of cell-mediated (CD4+ and CD8+ T cells, macrophages) as well as humoral immune response (by the formation of anti-HIV antibodies).
However, this period is short and the virus soon overpowers the host immune system.
Budding and release of virion:
Replicating viral particles are formed near the plasma membrane of the host CD 4+ T cell by the assembly of genomic RNA, HIV proteins and enzymes.
As these viral particles start budding from the host cell wall, the viral core acquires its envelope from the lipid bilayer of the host cell. A host protein, tetherin, may inhibit the release of budding viral particles from the host cell.
However, the action of tetherin of the host cell is counteracted by accessory viral protein Vpu (viral protein U). Thus, after budding, mature virions are released.
CD4+ T cell depletion:
HIV viraemia and depletion of CD4 + T cells in the host are correlated — the higher the number of copies of HIV, the more profound is the destruction of CD4 + T cells.
Possible mechanisms of destruction of CD4 + T cells are as under:
- Most commonly, this is due to direct virus-induced cytolysis of host CD4+ T cells.
- Loss of integrity of plasma membrane of the host cell during viral budding of viral particles may cause the death of host CD4+ T cells.
- HIV-infected CD4 + T cells may fuse to form syncytial giant cells which have abundant expression of gp120 and gp41 molecules and bind more and more uninfected CD4 + T cells.
- Syncytia are destined to die of apoptosis.
- Activation of inflammasome pathway (by the release of proinflammatory cytokines, may lead to death of CD4 + T cells by pyroptosis.
- Progressive destruction of the architecture of lymphoid tissue eventually leads to burnt-out lymphoid organs and a depleted lymphoid population in these organs.
Other indirect mechanisms of CD4 + T cells depletion are aberrant intracellular signalling, T cell activation-induced cell death, and autoimmune destruction of T cells.
Viral dissemination:
Release of viral particles from infected host cells spreads the infection to more CD4+ host cells and produces viraemia. Through circulation, the virus gains entry to the lymphoid tissues (lymph nodes, spleen) where it multiplies further; thus these tissues become the dominant site of the virus reservoir rather than circulation.
Impact of HIV infection on other immune cells:
HIV infects other cells of the host immune system and also affects non-infected lymphoid cells:
- Low levels of antigen-presenting cells These cells are circulating monocytes, macrophage in tissues and follicular dendritic cells of lymph nodes.
- HIV-infected monocytes-macrophages do not get destroyed but instead, become a reservoir of HIV infection.
- Infected dendritic follicular cells of the lymph nodes cause massive enlargement of follicle centres and account for persistent generalised lymphadenopathy in AIDS.
- Non-infected lymphoid cells These cells include B cells, NK cells and CD8+ T cells:
- B cells do not have receptors for HIV but the number of B cells slowly declines, their function of immunoglobulin synthesis is impaired due to a lack of their activation by depleting CD4+ T cells, and there may be non-specific hypergammaglobulinaemia.
- NK cells are also reduced due to a lack of cytokines from depleted CD4+ T cells.
- CD8+ cells show lymphocytosis but the cells having intact function of ADCC are reduced, possibly due to quantitative loss of CD4+ T cells and their qualitative dysfunction (reversal of CD4+ T cells: CD8+ T cell ratio).
The net result of immunological changes in the host due to HIV infection leads to profound immunosuppression rendering the host susceptible to opportunistic infections and tumours, to which the patient ultimately succumbs.
3. Hiv Infection Of the Central Nervous System:
Out of non-lymphoid organ involvement, HIV infection of nervous system (CNS) presenting with various neurocognitive deficits is the most serious.
About 75-90% of AIDS patients may demonstrate some form of neurological involvement at autopsy. It has also been suggested that CNS may serve as a sequestered organ for HIV that escapes attack by ART.
HIV infects microglial cells via infected macrophages but does not directly infect neurons. Recruitment and activation of macrophages by chemokines plays a key role in CNS manifestations of HIV infection i.e. it depicts M-tropism.
Alternate pathogenic mechanisms of neurologic manifestations in HIV infection of CNS are direct damage to neurons by HIV, or indirect damage by viral products, cytokines and other soluble factors produced by damaged neuroglial cells. A summary of major abnormalities in the immune system in AIDS.
Major abnormalities in the immune system in AIDS:
- T Cell abnormalities:
- Lymphopenia
- CD4+ T cell depletion
- Selective loss of CD4+ memory T cells
- CD8+ T cell lymphocytosis
- Reversal of CD4: CD8 cell ratio
- Decreased production of cytokines (IL-2, IFN-γ) by CD4+ T cells
- Decreased ADCC by CD8+ T cells
- Cell Abnormalities:
- Not infected, no viral damage
- Polyclonal activation with hypergammaglobulinaemia
- Circulating immune complexes
- Impaired antibody response to newer antigens
- Decreased antibody production due to decreased T helper Cell function
- Nk Cell Abnormalities:
- Not infected, no viral damage
- Depressed number, impaired function
- Increased iNKRs, decreased cytotoxicity
- Monocyte-Macrophage Cell Abnormalities:
- No Destruction
- Decreased chemotaxis
- Decreased phagocytosis
- Decreased HLA class II expression
- Decreased antigen presentation
Natural History Of HIV Infection
HIV infection progresses from an early acute syndrome to a prolonged asymptomatic state to a full-blown advanced disease as AIDS.
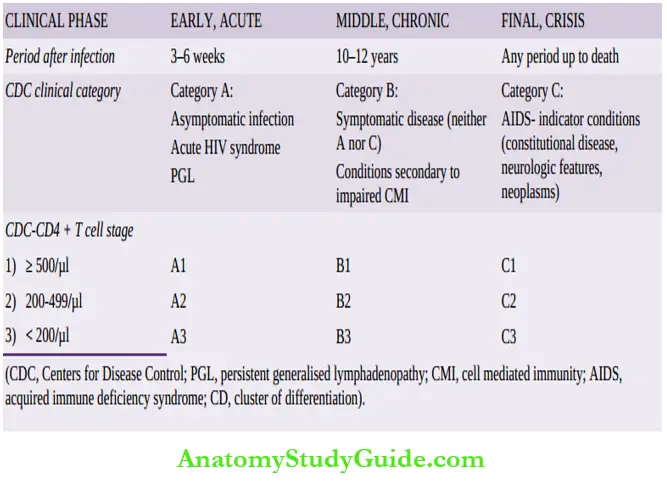
Thus, there are different clinical manifestations at different stages. Generally, in an immunocompetent host, the biologic course passes through the following 3 phases:
- Acute HIV syndrome: Entry of HIV into the body is heralded by the following sequence of events:
- High levels of plasma viraemia due to replication of the virus.
- Virus-specific immune response by formation of anti-HIV antibodies (seroconversion) after 3-6 weeks of initial exposure to HIV.
- Initially, sudden marked reduction in CD4+ T cells (helper T cells) followed by a return to normal levels.
- Rise in CD8+ T cells (cytotoxic T cells).
- The appearance of self-limited non-specific acute viral illness (flu-like or infectious mononucleosis-like) in 50-70% of adults within 3-6 weeks of initial infection.
- Manifestations include sore throat, fever, myalgia, skin rash, and sometimes, aseptic meningitis.
- These symptoms resolve spontaneously in 2-3 weeks.
- Middle chronic phase: Initial acute seroconversion illness is followed by a phase of competition between HIV and the host immune response:
- Viraemia due to viral replication in the lymphoid tissue continues which is initially not as high but with the passage of time viral load increases due to crumbling host defenses.
- The chronic stage, depending upon the host immune system, may continue as long as 10 years.
- Although CD 4+ T cells continue to proliferate their destruction by HIV is much more; the net result is a moderate fall in CD4+ T cell counts.
- Cytotoxic CD8+ T cell count remains high.
- Clinically, it may be a stage of latency and the patient may remain asymptomatic, or may develop mild constitutional symptoms and persistent generalised lymphadenopathy.
- Final crisis phase: This phase is characterised by profound immunosuppression and the onset of full-blown
AIDS and has the following features:
-
- A marked increase in viraemia.
- The time period from HIV infection through the chronic phase into full-blown AIDS may last 7- 10 years and culminate in death.
- CD 4+ T cells are markedly reduced (below 200 per µl). The average survival after the onset of full-blown AIDS of an untreated case is about 2 years.
Children often have a rapidly progressive disease and full-blown AIDS occurring at 4 to 8 years of age.
Classification Systems of HIV Infection:
Currently, there are two major staging and classification systems for HIV infection: US Centers for Disease Control and Prevention (CDC) (2013), and WHO Clinical Staging Disease
Classification System (2007). While the WHO system is based on clinical manifestations only, the CDC classification system is more acceptable since it takes into account 2 parameters: clinical manifestations and CD4+ T cell counts.
According to this system, irrespective of the presence of symptoms, any HIV-infected individual having a CD4+ T cell count of <200/µl is labelled as Aids
CDC defining criteria divide HIV infection into 5 clinical categories: 0, 1, 2, 3 and unknown.
- Stage 0: If after the first diagnosis of HIV infection, the test becomes negative within 6 months.
- Unknown: When clinical AIDS-defining criteria are present, but CD4 +T cell counts are not available.
- Advanced HIV disease (AIDS): Has 3 categories: A, B, C. Based on CD4+ T cell count, each of these is further subdivided into three CD stages 1, 2 and 3:
- CDC stage 1: CD4+ T cell count >500/µl
- CDC stage 2: CD4+ T cell count 200-499/µl
- CDC stage 3: CD4+ T cell count <200/µl)
Thus, combining clinical category and stage of CD4+ T cell count, there are 3 categories in each stage:
- A1, B1, C1
- A2, B2, C2
- A3, B3, C3
Criteria of clinical manifestations for each category are as under:
- Clinical category A: Includes a variety of conditions: asymptomatic case, persistent generalised lymphadenopathy (PGL), and acute HIV syndrome.
- Clinical category B: Includes symptomatic cases and includes conditions secondary to impaired cell-mediated immunity for example, Diarrohea lasting > one month, bacillary angiomatosis, mucosal candidiasis, fever, oral hairy leukoplakia, herpes zoster,
- ITP, pelvic inflammatory disease, peripheral neuropathy, cervical dysplasia and carcinoma in situ cervix etc.
- Clinical category C: This category includes conditions listed for AIDS surveillance case definition.
- These include Mucosal candidiasis, recurrent bacterial infections ( for example, Pneumonias), mycobacterial infection ( for example, Tuberculosis, Mycobacterium avium intracellulare complex), fungal infections ( for example, Histoplasmosis), parasitic infections ( for example, Pneumocystis jirovecii pneumonia), malnutrition and wasting of muscles etc.
CDC classification system (2013) for HIV-infected adults and adolescents:
Similarly, there are revised parameters for paediatric HIV classification in which area adjusted CD4+ T cell counts are given that are relatively higher in each corresponding category.
Pathological Lesions And Clinical Manifestations Of Hiv-Aids:
HIV/AIDS affects all body organs and systems. In general, clinical manifestations and pathological lesions in different organs and systems are owing to the progressive deterioration of the body’s immune system.
Disease progression occurs in all untreated patients, even if the disease is apparently latent. Antiretroviral treatment blocks and slows the progression of the disease.
Pathological lesions and clinical manifestations in HIV disease can be explained by 4 mechanisms:
- Due to viral infection directly The major targets are the immune system, central nervous system and lymph nodes (persistent generalised lymphadenopathy).
- Due to opportunistic infections Deteriorating immune system provides the body an opportunity to harbour microorganisms. A list of common opportunistic infectious agents affecting HIV/AIDS is given in.
- Due to secondary tumours, the End-stage of HIV/AIDS is characterised by the development of certain secondary malignant tumours.
- Due to drug treatment Drugs used in the treatment of HIV/AIDS and its complications produce toxic effects.
- These drugs include antiretroviral treatment, aggressive treatment of opportunistic infections and tumours.
Based on the above mechanisms, salient clinical features and pathological lesions in different organs and systems are briefly outlined below.
However, it may be mentioned here that many of the pathological lesions given below may not become clinically apparent during life and may be noted at autopsy alone.
- Wasting syndrome: Most important systemic manifestation corresponding to the body’s declining immune function is wasting syndrome defined as ‘involuntary loss of body weight by more than 10%’. It occurs due to multiple factors such as malnutrition, increased metabolic rate, malabsorption, anorexia, and ill effects of multiple opportunistic infections.
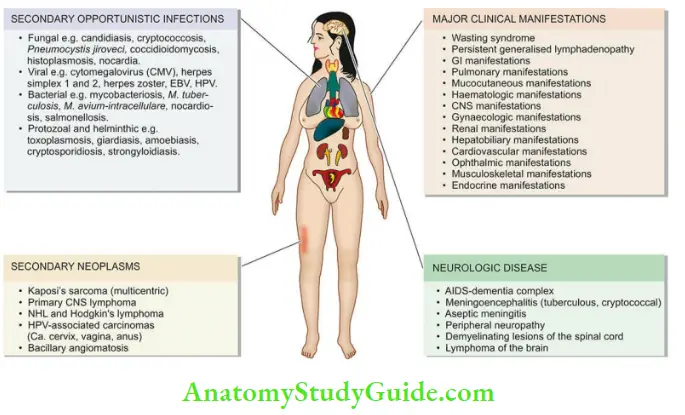
- Persistent generalised lymphadenopathy: In the early asymptomatic stage during the course of the disease, some patients may develop persistent generalised lymphadenopathy (PGL). PGL is defined as the presence of enlarged lymph nodes >1 cm at two or more extrainguinal sites for >3 months without an obvious cause.
- There is marked cortical follicular hyperplasia, due to proliferation of CD8+ T cells, B cells and dendritic follicular histiocytes.
- HIV-infected CD4+ T cells are seen in the mantle zone.
- In advanced cases of AIDS, lymph nodes show progressive depletion of lymphoid cells, or there may be an occurrence of opportunistic infection (for example,M. avium intracellulare, Histoplasma, Toxoplasma) or appearance of secondary tumours in the lymphoid tissue ( for example, Kaposi’s sarcoma, lymphoma).
- GI lesions and manifestations: Almost all patients with HIV infection develop gastrointestinal manifestations.
- These include: chronic watery or bloody diarrhoea, oral, oropharyngeal and oesophageal candidiasis, anorexia, nausea, vomiting, mucosal ulcers, abdominal pain.
- These features are due to opportunistic infections ( for example,Candida, Clostridium,
- Shigella, Salmonella, Giardia, Entamoeba histolytica, Cryptosporidum, CMV).
- Advanced cases may develop secondary tumours in GIT ( for example, Kaposi’s sarcoma, lymphoma).
- Pulmonary lesions and manifestations: Symptoms pertaining to the lungs develop in about 50- 75% of cases and are a major cause of death in HIV/AIDS.
- These features are largely due to opportunistic infections causing pneumonia e.g. with Pneumocystis jiroveci (earlier called P. carinii), M. tuberculosis, CMV, Histoplasma, and Staphylococci. Lung abscess too may develop.
- Other pulmonary manifestations include adult respiratory distress syndrome and secondary tumours ( for example, Kaposi’s sarcoma, lymphoma).
- Mucocutaneous lesions and manifestations: Symptoms due to mucocutaneous involvement occur in about 50–75% of cases.
- Mucocutaneous viral exanthem in the form of erythematous rash is seen at the onset of the primary infection itself.
- Other mucocutaneous manifestations are allergic (for example, Drug reaction, seborrhoeic dermatitis), infectious (viral infections such as herpes, varicella zoster, EB virus, HPV; bacterial infections such as M. avium intracellulare, Staph. aureus; fungal infections such as Candida, Cryptococcus, Histoplasma) and neoplastic ( for example, Kaposi’s sarcoma, squamous cell carcinoma, basal cell carcinoma, cutaneous lymphoma).
- Haematologic lesions and manifestations: Involvement of the haematopoietic system is common during the course of HIV/AIDS.
- These include anaemia, leucopenia, and thrombocytopenia.
These changes are due to bone marrow suppression from several mechanisms: infectious involvement for example,By HIV, mycobacteria, fungi, and parvoviruses, or by lymphomatous involvement.
- These include anaemia, leucopenia, and thrombocytopenia.
- CNS lesions and manifestations: Neurological manifestations occur in almost all cases during the course of disease and are an important cause of mortality and morbidity.
- These may be inflammatory, demyelinating and degenerative conditions. HIV encephalopathy or AIDSassociated dementia complex is an AIDS-defining condition and manifests clinically with, deteriorating neurocognitive symptoms, commonly termed as HAND (HIV-associated neurocognitive disorder).
- Other pathological lesions in HIV/AIDS are meningitis (tuberculous, cryptococcal) demyelinating lesions of the spinal cord, and peripheral neuropathy and lymphoma of the brain.
- Gynaecologic lesions and manifestations: Gynaecologic symptoms are due to monilial (candidal) vaginitis, cervical dysplasia, carcinoma cervix, and pelvic inflammatory disease.
- Renal lesions and manifestations: Features of renal impairment may appear due to HIV-associated nephropathy and genitourinary tract infections including pyelonephritis.
- Hepatobiliary lesions and manifestations: Manifestations of the hepatobiliary tract are due to the development of coinfection with hepatitis B or C, due to the occurrence of other infections and due to drug-induced hepatic injury. The lesions include steatosis, granulomatous hepatitis and opportunistic infections (M. tuberculosis, Mycobacterium avium intracellulare, Histoplasma).
- Cardiovascular lesions and manifestations: Diseases affecting the heart are common autopsy findings and include a form of dilated cardiomyopathy called HIV-associated cardiomyopathy, pericardial effusion in advanced disease as a reaction to opportunistic infection, lymphoma and Kaposi’s sarcoma.
- Ophthalmic lesions: HIV-associated ocular manifestations occur from opportunistic infections ( for example, CMV retinitis), HIV retinopathy, and secondary tumours.
- Musculoskeletal lesions: These include osteoporosis, osteopenia, arthropathies, osteomyelitis and polymyositis.
- Endocrine lesions: Several metabolic derangements may occur during the course of the disease.
- Syndrome of lipodystrophy (buffalo hump) due to dyslipidaemia, hyperinsulinaemia and hyperglycaemia may occur.
- Besides, abnormality of thyroid function, hypogonadism and inappropriate release of ADH may be associated.
Lesions And Manifestations In Paediatric Aids
Children develop clinical manifestations of AIDS more rapidly than adults. Besides the development of opportunistic infections and tumours, neurologic impairment in children cause slowing of development and growth.
Diagnosis Of Hiv-Aids:
The investigations of a suspected case of HIV/AIDS are categorised into 3 groups:
- Tests for establishing HIV infection
- Tests for defects in immunity, and
- Tests for detection of opportunistic infections and secondary tumours.
However, usually, initial testing for antibodies is done against HIV by ELISA and confirmation is done by Western blot or immunofluorescence test. These tests are as under
1. Tests for establishing HIV infection: These include antibody tests and direct detection of HIV.
Antibody tests These tests are as under:
Serologic testing Initial screening is done by serologic test for antibodies by enzyme-linked immunosorbent assay (ELISA) against HIV antigen p 24 and has a sensitivity of >99.5%.
Nucleic acid testing (NAT) of blood against the p antigen has decreased the interval between infection and detection (window period) to 12 days.
Besides, ELISA may be false positive in autoantibodies, liver disease, recent vaccination against flu, and other viral infections.
If ELISA is positive or indeterminate, it is repeated.
HIV Western blot If ELISA is positive on repeat testing, confirmation is done by Western blot for the presence of specific antibodies against all three major gens of HIV: gag, pol and env.
Tests for diagnosis of HIV/AIDS:
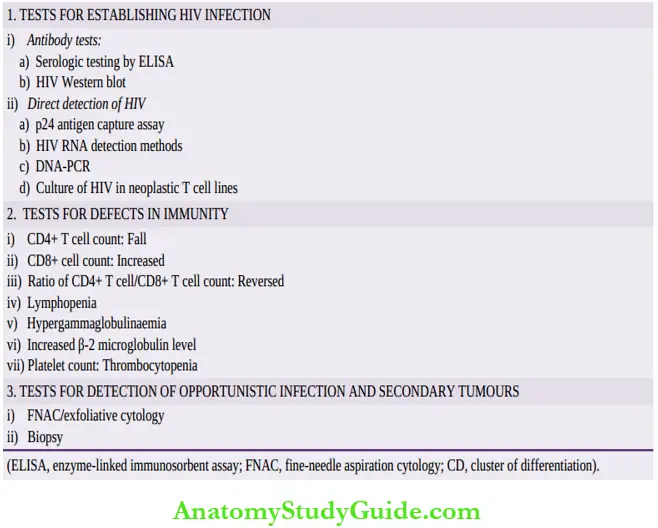
Direct detection of HIV These tests are as follows:
- p24 antigen capture assay.
- HIV RNA detection methods: by RT-PCR (reverse trasnscriptase polymerase chain reaction), branched DNA, TMA (transcription-mediated amplification), NASBA or NucleiScan (nucleic acid sequence-based amplification).
- DNA-PCR by amplification of proviral DNA.
- Culture of HIV from blood monocytes and in neoplastic T cell lines.
2. Tests for defects in immunity:
These tests are used for diagnosis as well as for monitoring the treatment of cases.
- CD4+ T cell counts Progressive fall in a number of CD4+ T cells is of paramount importance in diagnosis and staging as per CDC categories described above.
- Rise in CD8+ T cells.
- Reversal of CD4+ to CD8+ T cell ratio.
- Lymphopenia.
- Polyclonal hypergammaglobulinaemia.
- Increased β2 microglobulin levels.
- Platelet count revealing thrombocytopenia.
3. Tests for detection of opportunistic infections and secondary tumours:
Diagnosis of organs involved in opportunistic infection and specific tumours secondary to HIV/AIDS is made by aspiration or biopsy methods as for the corresponding primary disease.
Acquired Immunodeficiency Syndrome (AIDS)
AIDS is defined as an acquired infection caused by as determined by two parameters: specific clinical manifestations of HIV-AIDS and decreased CD4+ T cell counts.
AIDS has pandemic distribution and is seen in all continents. As per current estimates, 37 million people are living with AIDS globally, countries in the African continent have the largest number of cases.
It is caused by retrovirus, HIV-1 or HIV-2, the former being a much more common etiologic agent in most parts of the world.
Routes of spread of infection are: sexual (both homo- and heterosexual), via blood route and by use of contaminated blood products, perinatal transmission to the newborn from infected mothers, needle stick injuries, and rarely from other body fluids.
Mechanism of acquiring disease is by selective tropism of HIV for CD4 molecule located on CD4+ helper T cells most importantly, and other cells (monocyte-macrophages, neuroglia). The virus internalises in the target cell, uncoats and forms double-stranded viral DNA, which then integrates into the host cell DNA, forming HIV provirus.
Virus replicates inside the cytoplasm and ultimately destroys the host CD4+T cells. The viral particles then disseminate to the other lymphoid tissues and CNS.
- As per CDC, HIV is defined by clinical features and by CD4+ T cell count:
- CDC CD4+ T cell count stages are : stage 1 CD4 + T cell count >500/µl, stage 2 CD4 +
- T cell counts 200-499/µl, and stage 3 CD4+ T cell count <200/µl.
CDC clinical categories are: A = asymptomatic case, acute HIV syndrome or with PGL, B=symptomatic case, and secondary conditions due to impaired CMI, and C=having AIDS indicator conditions.
AIDS involves multiple systems and affects almost all organs. The main pathologic lesions in full-blown case are due to opportunistic infections, secondary tumours and CNS manifestations.
Diagnosis of AIDS is made by tests to establish infection (antibody testing by ELISA, confirmation by HIV Western blot, and direct detection of HIV RNA), and to detect defects in immunity (low CD4 cell counts, reversal of CD4+: CD8+ cells). Diagnosis of
AIDS-associated opportunistic infections and tumours are made by traditional methods.
Leave a Reply