Autoimmune Diseases
A normal immune system has an inbuilt capacity to distinguish ‘self’ from ‘non-self’ or foreign tissues; when confronted with a foreign invading agent, the host normally mounts an inflammatory response without causing harm to self-tissues.
Table of Contents
Autoimmune diseases, on the other hand, are essentially due to failure of such distinction between ‘self’ and ‘non-self’; instead the body reacts by immunologic reaction against its own tissues and causes tissue damage.
Read And Learn More Amyloidosis
Autoimmunity refers to mere presence of either autoantibodies or T cell self-reactivity against own tissues, both of which may or not cause tissue damage, and thus not always pathogenic.
In other words, loss of tolerance to one’s own tissues is autoimmunity i.e. autoimmunity is the opposite of immune tolerance. Therefore, before discussing types and examples of autoimmune diseases, a basic concept of immune tolerance is given below, followed by mechanisms of autoimmunity.
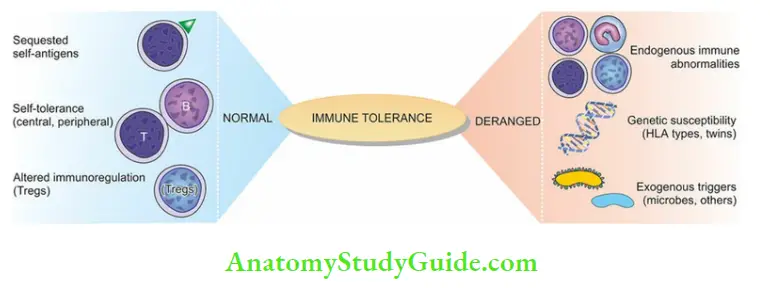
Immune Tolerance
Immune tolerance is a normal phenomenon present since fetal life; it is defined as the ability of an individual to recognize autoantigens and show selective unresponsiveness to autoantigens.
Although clones of lymphoid cells capable of responding to autoantigens exist in normal individuals, these cells respond selectively to autoantigens. This is based on three general processes:
- Sequestration of self-antigens
- Generation and maintenance of self-tolerance, and
- Altered immunomodulatory controls.
A derangement of any of these normal processes predisposes to autoimmunity.
1. Sequestration Of Self-Antigens:
Some self-antigens reside at locations that are hidden from the immune system at ‘privileged sites’ which are out of circulatory reach of blood or lymph, for example, Anterior chamber of the Eye, or Brain.
Thus, the lymphoid system remains in a state of ‘immune ignorance’ about these sites. Besides, there is the production of immunosuppressive cytokines ( for example, TGF-β) locally at these sites, causing apoptosis of activated T lymphocytes.
2. Generation And Maintenance Of Self-Tolerance:
A state of self-tolerance is generated and maintained by one of the two mechanisms: central deletion of autoreactive lymphocytes, and peripheral silencing of autoreactive lymphocytes (or clonal energy).
- Central Deletion of Auto-reactive Lymphocytes:
- During fetal development, clones of self-reacting T and B cells recognize self-antigens during their maturation in corresponding lymphoid organs — T cells in the thymus and B cells in the bone marrow.
- During the maturation of B and T cells in these central lymphoid organs, such ‘forbidden clones’ of self-reacting lymphoid cells are eliminated by apoptosis; hence this is also called central tolerance.
Peripheral Silencing of Auto-reactive Lymphocytes (Clonal Energy):
These are mechanisms that put the auto-reactive lymphocytes in the peripheral tissues to inactivation mode. The term clonal energy is defined as a state in which lymphocytes have the ability to recognize self-antigens but fail to show immune response even after a prolonged duration. It mainly affects T cells although B cells may also develop clonal energy:
- T cell clonal energy: Normally, antigen-specific T lymphocytes can be activated after they have been processed as under:
- Recognition of a peptide antigen on T cell surface after it is presented to T cell by MHC molecule on antigen-presenting dendritic cells.
- A co-stimulatory signal (CD28) provided by antigen-presenting cells. However, when antigen is presented to T cells by antigen-presenting cells either without or a weak costimulatory signal by CD28 molecule, it leads to T cell clonal anergy.
- B cell clonal anergy: When helper T cells fail to provide activation to B cells, B lymphocytes are unable to respond to self-antigen in the peripheral tissues. Alternatively, inhibitory receptors on B cells may inactivate B cell function.
3. Altered Immunomodulatory Controls:
A subset of T cells called regulatory T cells (Tregs), normally act to regulate T cell function and thus prevent T cell attack on self-antigen. A downregulation of Tregs function is mediated by the secretion of cytokines (IL-4, IL-10, TGF-β) by CD4+ helper TH2 cells which are a form of Treg cells.
In support of the role of Tregs is the pathogenesis of IPEX (immunodysregulation, polyendocrinopathy, enteropathy X-linked) syndrome which is due to failure in expression of FOXP3 gene that encodes for differentiation of Treg cells.
Leave a Reply