Blood Vessels And Lymphatics
Normal Structure:
Table of Contents
Arteries:
Depending upon the calibre and certain histologic features, arteries are divided into 3 types:
Large (elastic) arteries, medium-sized (muscular) arteries and the smallest arterioles.
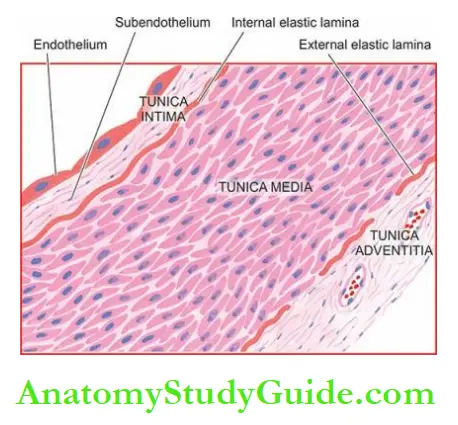
Histologically, all major arteries of the body have 3 layers in their walls: the tunica intima, the tunica media and the tunica adventitia.
These layers progressively decrease with a diminution in the size of the vessels.
Read And Learn More: Systemic Pathology Notes
1. Tunica intima: This is the inner coat of the artery.
It is composed of the lining endothelium, and subendothelial connective tissue and is bounded externally by internal elastic lamina.
- The endothelium is a layer of flattened cells adjacent to the flowing blood. Narrow junctions exist between the adjoining endothelial cells through which certain materials pass. The integrity of the endothelial layer is of paramount importance in the maintenance of vascular functions since damage to it is the most important event in the initiation of thrombus formation at the site.
- Subendothelial tissue consists of a loose meshwork of connective tissue that includes minimal cells, collagen, proteoglycans, elastin and matrix glycoproteins.
- Internal elastic lamina is a layer of elastic fibres having minute fenestrations.
2. Tunica media: Tunica media is the middle coat of the arterial wall, bounded internally by internal elastic lamina and externally by external elastic lamina.
- This layer is the thickest and consists mainly of smooth muscle cells and elastic fibres.
- The external elastic lamina consisting of condensed elastic tissue is less well-defined than the internal elastic lamina.
3. Tunica adventitia: Outer coat of arteries is the tunica adventitia.
- It consists of a loose mesh of connective tissue and some elastic fibres that merge with the adjacent tissues. This layer is rich in lymphatics and autonomic nerve fibres.
The layers of the arterial wall receive nutrition and oxygen from 2 sources:
1. Tunica intima and inner third of the media are nourished by direct diffusion from the blood present in the lumen
2. Outer two-thirds of the media and the adventitia is supplied by vasa vasa (i.e. vessels of vessels), the nutrient vessels arising from the parent artery.
As the calibre of the artery decreases, the three layers progressively diminish.
Thus, there are structural variations in three types of arteries:
- Large, elastic arteries such as the aorta, innominate, common carotid, major pulmonary, and common iliac arteries have a very high content of elastic tissue in the media and thick elastic laminae and hence the name.
- Medium-sized, muscular arteries are the branches of elastic arteries. All three layers of the arterial wall are thinner than in the elastic arteries. The internal elastic lamina appears as a single wavy line while the external elastic lamina is less prominent. The media primarily consists of smooth muscle cells and some elastic fibres.
- Arterioles are the smallest branches with an internal diameter of 20-100 µm. Structurally, they consist of three layers as in muscular arteries but are much thinner and cannot be distinguished. The arterioles consist of a layer of endothelial cells in the intima, one or two smooth muscle cells in the media and a small amount of collagen and elastic tissue comprising the adventitia. The elastic laminae are virtually lost.
Veins:
- The structure of normal veins is basically similar to that of arteries.
- The walls of the veins are thinner, the three tunicates (intima, media and adventitia) are less clearly demarcated, and elastic tissue is scanty and not clearly organised into internal and external elastic laminae.
- The media contains a very small amount of smooth muscle cells with abundant collagen.
- All veins, except vena cavae and common iliac veins, have valves best developed in veins of the lower limbs.
- The valves are delicate folds of the intima, located every 1-6 cm, often next to the point of entry of a tributary vein.
- They prevent any significant retrograde venous blood flow.
Capillaries:
- Capillaries are about the size of an RBC (7-8 µm) and have 1-2 endothelial cells but no media.
- Blood from capillaries returns to the heart via post-capillary venules and from there into venules and then drained into veins.
Lymphatics:
- Lymphatic capillaries, lymphatic vessels and lymph nodes comprise the lymphatic system.
- Lymphatic capillaries resemble blood capillaries, and larger lymphatics are identical to veins.
- However, lymphatics lined by a single layer of endothelium have thin muscle in their walls than in veins of the same size and the valves are more numerous.
- Lymphatic capillaries and lymphatics form plexuses around tissues and organs.
- The walls of lymphatic capillaries are permeable to tissue fluid, proteins and particulate matter.
Normal Structure:
- Arteries are of 3 types: large (elastic) arteries, medium-sized (muscular) arteries and the smallest arterioles.
- Major arteries of the body have 3 layers in their walls: the tunica intima, tunica media and tunica adventitia.
- Internal and external elastic laminae bounding the media on either side are well-developed in arteries.
- The walls of the veins are thinner, three tunicates (intima, media and adventitia) are less clearly demarcated, and elastic tissue is scanty and not clearly organised into internal and
external elastic laminae. - Capillaries are tiny the size of a red cell and have 1-2 endothelial cells only in their wall.
- Lymphatic channels are similar to veins and form plexus around tissues and organs
Arteriosclerosis
Arteriosclerosis is a general term used to include all conditions with thickening and hardening of the arterial walls due to degenerative changes.
The following morphologic entities are included under arteriosclerosis:
- Senile arteriosclerosis (affects arteries)
- Hypertensive arteriolosclerosis (affects arterioles)
- Monckeberg’s arteriosclerosis (Medial calcific sclerosis) (affects arteries)
- Atherosclerosis (affects arteries) The most common and most important form of arteriosclerosis is atherosclerosis; if not specified, the two terms are used interchangeably with each other.
Senile Arteriosclerosis:
Senile arteriosclerosis is the thickening of media and intima of the arteries seen due to ageing.
The changes are non-selective and affect most of the arteries. These are possibly induced by stress and strain on vessel walls during life.
Morphologic Features:
The changes are as under:
- Fibroelastosis The intima and media are thickened due to an increase in elastic and collagen tissue.
- Elastic reduplication The internal elastic lamina is split or reduplicated so that two wavy
layers are seen.
Eventually, the fibrotic changes result in age-related elevation of systolic blood pressure.
Hypertensive Arteriolosclerosis:
- Hypertension is the term used to describe an elevation in blood pressure.
- Pathology of 3 forms of hypertension—systemic, pulmonary and portal, is discussed in detail with diseases of the kidneys, lungs and liver respectively.
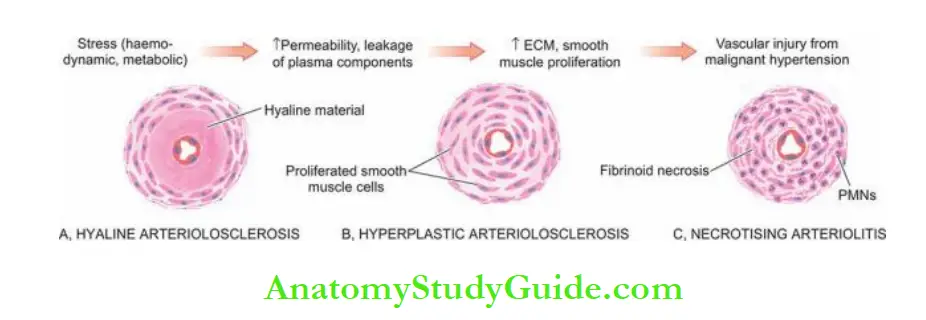
- Hypertensive arteriolosclerosis is the term used to describe 3 morphologic forms of vascular disease affecting arterioles and small muscular arteries.
- These are hyaline arteriolosclerosis, hyperplastic arteriolosclerosis and necrotising arteriolitis.
All three types are common in hypertension but may occur due to other causes such as diabetes as well:
1. Hyaline arteriolosclerosis is a common arteriolar lesion that may be seen physiologically due to ageing, or may occur pathologically in benign nephrosclerosis in hypertensives and
as a part of microangiopathy in diabetics.
2. Hyperplastic (or proliferative) arteriolosclerosis is a characteristic lesion of malignant hypertension; other causes include haemolytic-uraemic syndrome, scleroderma and toxaemia of pregnancy.
3. Necrotising arterioles are seen in cases of severe hypertension and malignant hypertension.
In this, either some parts of small arteries and arterioles have changes of hyaline or hyperplastic arteriolosclerosis and some parts show necrosis, or necrosis may be superimposed on hyaline or hyperplastic arteriolosclerosis.
Pathogenesis:
The following sequence in the evolution of pathologic lesions of hypertensive arteriolosclerosis is proposed:
- There is an increase in the permeability of the vessel wall due to haemodynamic stress in hypertension and metabolic stress in diabetes.
- This results in the insulation of plasma-derived components across the leaky vascular endothelium.
- This is substantiated by the demonstration of immunoglobulins, complement, fibrin and lipids in the lesions (hyaline arteriolosclerosis).
- Simultaneously, there is increased synthesis of smooth muscle matrix and extracellular matrix, followed by proliferation of smooth cells in the media and reduplicated basement membrane (hyperplastic arteriolosclerosis).
- About 5% of cases of hypertension progress to malignant hypertension in which there is sudden and great elevation of pressure.
- This causes direct physical injury to the vessel wall followed by fibrinoid necrosis and inflammation in the vessel wall (necrotising arteritis).
- The change may superimpose on hyaline arteriolosclerosis or proliferative arteriolosclerosis (i.e. proliferative arterioles).
Morphologic Features:
The changes in three forms of arteriosclerosis are as under:
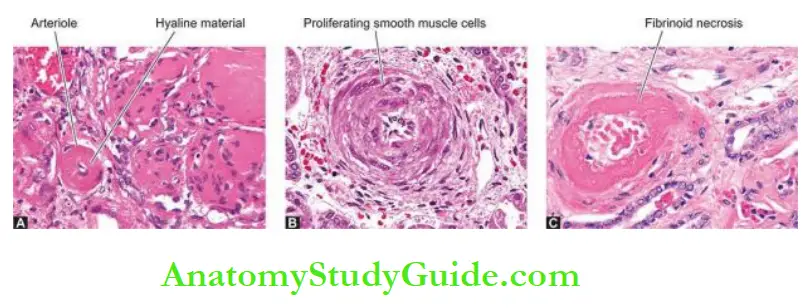
1. Hyaline Arteriolosclerosis: The visceral arterioles are particularly involved.
- The vascular walls are thickened and the lumina narrowed or even obliterated.
- Microscopically, the thickened vessel wall shows structureless, eosinophilic, hyaline material in the intima and media.
2. Hyperplastic Arteriolosclerosis: The morphologic changes affect mainly the media, especially the interlobular arteries in the kidneys.
Microscopically: thickened vascular wall shows the following changes:
- The onion-skin lesion consists of loosely-placed concentric layers of hyperplastic smooth muscle cells of media like the bulb of an onion.
- The basement membrane is also thickened and reduplicated.
- Mucinous intimal thickening is the deposition of amorphous ground substance, probably proteoglycans, with scanty cells.
- Fibrous intimal thickening is less common and consists of bundles of collagen, elastic fibres and hyaline deposits in the intima. Severe sclerosis results in narrowed or obliterated lumen. With time, the lesions become more and more fibrotic.
3. Necrotising Arteriolitis: Besides the changes of hyaline or hyperplastic arteriolosclerosis, there are superimposed changes of necrotising arterioles that include fibrinoid necrosis of the vessel wall and acute inflammatory infiltrate of neutrophils in the vessel wall.
Oedema and haemorrhages often surround the affected vessels.
Monckeberg’s Arteriosclerosis (Medial Calcific Sclerosis):
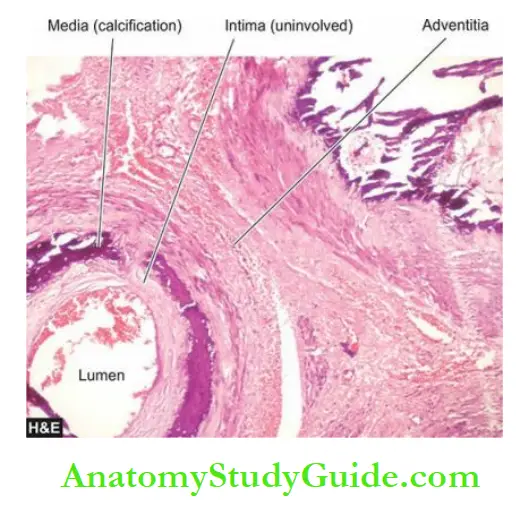
- Monckeberg’s arteriosclerosis is the calcification of the media of large and medium-sized muscular arteries, especially of the extremities and of the genital tract, in persons past the age of 50.
- The condition occurs as an age-related physiologic process of medial degeneration having dystrophic calcification.
- It has little or no clinical significance. However, excessive medial calcification also occurs in some pathological states like pseudoxanthoma elasticum and in idiopathic arterial calcification of infancy.
Morphologic Features:
- Medial calcification is often an incidental finding in X-rays of the affected sites having muscular arteries.
- The deposition of calcium salts in the media produces pipestem-like rigid tubes without causing the narrowing of the lumen.
Microscopically, Monckeberg’s arteriosclerosis is characterised by deposits of calcium salts in the media without associated inflammatory reaction while the intima and the adventitia are spared.
Often, coexistent changes of atherosclerosis are present altering the histologic appearance.
Arteriosclerosis:
- Arteriosclerosis is the term used for the thickening and hardening of the arterial wall.
- Senile arteriosclerosis is age-related thickening of the intima and media.
- Hypertensive arteriolosclerosis affects arterioles and includes hyaline thickening, hyperplastic or proliferative (onion-skin) change, and necrotising arterioles.
- These changes occur due to the insulation of plasma proteins and proliferated smooth muscle of media.
- Monckeberg’s medial calcific sclerosis affects the media of large and medium-sized muscular arteries as an age-related physiologic change.
- It is an example of dystrophic calcification in degenerated media.
Atherosclerosis:
Definition:
- Atherosclerosis is a thickening and hardening of large and medium-sized muscular arteries, primarily due to the involvement of tunica intima and is characterised by fibrofatty plaques or atheromas.
- The term atherosclerosis is derived from athero-(meaning porridge) referring to the soft lipid-rich material in the centre of atheroma, and sclerosis (scarring) referring to connective tissue in the plaques.
- Atherosclerosis is the commonest and the most important of the arterial diseases.
- Though any large and medium-sized artery may be involved in atherosclerosis, the most commonly affected are the aorta, the coronaries and the cerebral arterial systems.
Therefore, the major clinical syndromes resulting from ischaemia due to atherosclerosis are as under:
- Heart Angina and myocardial infarcts or heart attacks
- Brain Transient cerebral ischaemia and cerebral infarcts or strokes
- Peripheral arteries Peripheral arterial disease
- Other sequelae Aneurysmal dilatation due to a weakened arterial wall, chronic ischaemic heart disease, ischaemic encephalopathy and mesenteric arterial occlusion.
Aetiology:
Atherosclerosis is widely prevalent in industrialised countries.
However, the majority of the data on aetiology are based on animal experimental work and epidemiological studies.
The incidence of atherosclerosis quoted in the literature is based on the major clinical syndromes produced by it, the most important interpretation being that death from myocardial infarction is related to underlying atherosclerosis.
Cardiovascular disease, mostly related to atherosclerotic coronary heart disease or ischaemic heart disease (IHD) is the most common cause of premature death in the developed countries of the world.
- It is estimated that by the year 2020, cardiovascular disease, mainly atherosclerosis, will become the leading cause of total global disease burden.
- Systematic large-scale studies of investigations on living populations have revealed a number of risk factors which are associated with an increased risk of developing clinical atherosclerosis.
- Often, they are acting in combination rather than singly. These risk factors are divided into three groups:
- Major risk factors modifiable by lifestyle and/or therapy These are those risk factors which pose a definite coronary risk and can be controlled by modifying lifestyle or by pharmacotherapy, or both.
- These are lipid disorders (dyslipidaemia), hypertension, diabetes mellitus, smoking and lifestyle risk factors (atherogenic diet, obesity, physical inactivity).
- Constitutional non-modifiable risk factors These are those risk factors which are constitutional for an individual and thus are not modifiable.
- These are increasing age, male sex, genetic abnormalities, and familial and racial predisposition.
- Non-traditional emerging risk factors and biomarkers These are factors which either minimally contribute to atherosclerosis or are used as biomarkers for risk assessment.
- Apparently, a combination of etiologic risk factors has an additive effect in producing the lesions of atherosclerosis.
1. Major Risk Factors Modifiable By Lifestyle And/Or Therapy
- Dyslipidaemia (High LDL, low HDL cholesterol)
- Hypertension
- Diabetes mellitus
- Smoking
- Lifestyle risk factors (atherogenic diet, obesity, physical inactivity)
2. Constitutional Non-Modifiable Risk Factors
- Age
- Sex
- Genetic factors
- Familial and racial factors
3. Non-Traditional Emerging Risk Factors
- Environmental influences
- Oestrogen hormone
- Stressful behavioural pattern
- Hyperhomocysteinaemia
- Homocystinuria
- Prothrombotic factors
- Infectious burden
- Excessive alcohol consumption
- Biomarkers for risk assessment
1. Major Risk Factors Modifiable By Life Style and/or Therapy:
1. Lipid Disorders (Dyslipidaemia): The most firmly established and best understood major risk factors for atherosclerosis are abnormalities in plasma lipoproteins and derangements in lipid metabolism.
Disorders of lipoprotein metabolism are collectively called dyslipidaemia. It has been firmly established that hypercholesterolaemia has a direct proportional relationship with atherosclerosis and IHD.
The following evidence supports this hypothesis:
- The atherosclerotic plaques contain cholesterol and cholesterol esters, largely derived from the lipoproteins in the blood.
- The lesions of atherosclerosis can be induced in experimental animals by feeding them with a diet rich in cholesterol.
- Individuals with hypercholesterolaemia due to various causes such as in diabetes mellitus, myxoedema, nephrotic syndrome, von Gierke’s disease, xanthomatosis and familial hypercholesterolaemia have an increased risk of developing atherosclerosis and IHD.
- Populations having hypercholesterolaemia have higher mortality from IHD.
- Dietary regulation and administration of cholesterol-lowering drugs have a beneficial effect on reducing the risk of IHD.
Lipoproteins are complexes of lipids and proteins which essentially transport cholesterol, triglycerides and fat-soluble vitamins.
Lipids are insoluble in blood and therefore are carried in circulation and across the cell membrane by carrier proteins called apoproteins.
Different apoproteins are named by letter A, B, C, D etc while their subfractions are numbered serially.
The lipoproteins are divided into classes according to the density of the solvent in which they remain suspended on centrifugation at high speed.
The major classes of lipoprotein particles are chylomicrons, very low-density lipoproteins (VLDL), low-density lipoproteins (LDL), and high-density lipoproteins (HDL). The concentration of cholesterol in the serum reflects the concentrations of different lipoproteins in the serum.
The major fractions of lipoproteins tested in blood lipid profile and their varying effects on atherosclerosis and IHD are as under:
Total cholesterol: Desirable normal serum level is 140-199 mg/dl, while levels between 200-240 mg/dl are considered borderline high.
An elevation of total serum cholesterol levels above 260 mg/dl in men and women between 30 and 50 years of age have a three times higher risk of developing IHD as compared with people with total serum cholesterol levels within normal limits.
Triglycerides: Normal serum level is below 150 mg/dl.
Low-density lipoproteins cholesterol (LDL-C) Optimal serum level of LDL-C is <100 mg/dl.
LDL is the richest in cholesterol and has the maximum association with atherosclerosis.
Very-low-density lipoprotein cholesterol (VLDL-C) VLDL-C carries much of the triglycerides and its blood levels therefore parallel with that of triglycerides; VLDL has a less marked effect than LDL.
High-density lipoproteins cholesterol (HDL-C) Normal desirable serum level of HDL-C is <50 mg/dl. HDL is protective (‘good cholesterol’) against atherosclerosis.
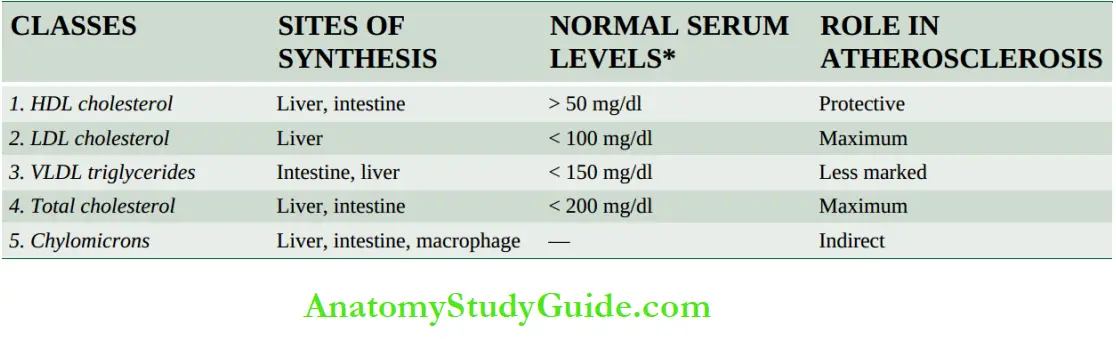
Lipids can also be measured in plasma (EDTA blood); plasma values are 3% lower than in serum.
(HDL, high-density lipoproteins; LDL, low-density lipoproteins; VLDL, very low-density lipoproteins).
Major mechanisms of dyslipidaemia from disordered lipoprotein metabolism are as under:
- Excessive hepatic secretion of triglyceride-rich VLDL
- Impaired lipolysis of triglyceride-rich lipoproteins
- Reduced hepatic uptake of receptor apoB-containing lipoproteins
- Combination of genetic predisposition and secondary factors in low HDL cholesterol.
- Currently, management of dyslipidaemia is directed at lowering LDL-C in particular, and total cholesterol in general, by use of statins, and for raising HDL-C by weight loss, exercise and use of nicotinic acid.
Thus, the preferred term for hyperlipidaemia is dyslipidaemia because the target of management is lowering one risky plasma lipoprotein cholesterol (i.e. LDL-C) when elevated, and raising the level of good plasma lipoprotein cholesterol (i.e. HDL-C) if it is low.
2. Hypertension: Hypertension is a risk factor for all clinical manifestations of atherosclerosis.
Hypertension doubles the risk of all forms of cardiovascular disease. It acts probably by mechanical injury to the arterial wall due to increased blood pressure.
Elevation of systolic pressure of over 160 mmHg or a diastolic pressure of over 95 mmHg is associated with a five times higher risk of developing IHD than in people with blood pressure within the normal range (120/80 mmHg or less).
Patients on antihypertensive medication also have an increased coronary risk than others.
3. Smoking: The extent and severity of atherosclerosis are much greater in smokers than in non-smokers.
Cigarette smoking is associated with a higher risk of atherosclerotic IHD and sudden cardiac death. Men who smoke a pack of cigarettes a day are 3-5 times more likely to die of IHD than non-smokers.
The increased risk and severity of atherosclerosis in smokers are due to reduced levels of HDL, a deranged coagulation system and accumulation of carbon monoxide in their blood that produces carboxyhaemoglobin and eventually hypoxia in the arterial wall favouring
atherosclerosis.
4. Diabetes Mellitus: Clinical manifestations of atherosclerosis are far more common and develop at an early age in people with both type 1 and type 2 diabetes mellitus.
In particular, an association of type 2 diabetes mellitus characterised by insulin resistance and abnormal lipid profile termed ‘diabetic dyslipidaemia’ (high LDL-C, low HDL-C, elevated triglycerides) is common and heightens the risk of cardiovascular disease.
The risk of developing IHD is doubled, the tendency to develop cerebrovascular disease is high, and the frequency to develop gangrene of the foot is about 100 times increased.
The causes of increased severity of atherosclerosis are complex and numerous which include endothelial dysfunction, increased aggregation of platelets, increased LDL-C and decreased HDL-C.
A combination of diabetic dyslipidaemia, hypertension, obesity and insulin resistance is termed ‘metabolic syndrome’ which poses a still greater risk to IHD.
5. Lifestyle Risk Factors: These are some risk factors which are preventable causes of atherosclerosis and can be modified by lifestyle changes.
These are as under:
- Atherogenic diet Many studies have demonstrated the harmful effect of a diet containing larger quantities of saturated fats. (e.g. in eggs, meat, milk, butter etc) and trans fats (i.e. unsaturated fats produced by artificial hydrogenation of polyunsaturated fats) which raise the plasma cholesterol level.
- On the contrary, a diet low in saturated fats and high in poly-unsaturated fats and having omega-3 fatty acids (e.g. in fish, fish oils etc) lowers the plasma cholesterol levels. Aside from a lipid-rich diet, a high intake of the total number of calories from carbohydrates, proteins, alcohol and sweets has adverse effects.
- Obesity and insulin resistance frequently coexist and are accompanied by dyslipidaemia. A body mass index (BMI) of 30 kg/m2 or more is associated with coronary risk due to atherosclerosis.
- Physical inactivity Sedentary lifestyle and lack of exercise are associated with the risk of developing atherosclerosis and its complications.
2. Constitutional Non-Modifiable Risk Factors:
1. Age: Atherosclerosis is an age-related disease. Though early lesions of atherosclerosis may be present in childhood, clinically significant lesions are found with increasing age.
Fully developed atheromatous plaques usually appear in the 4th decade and beyond. Evidence in support comes from the high death rate from IHD in this age group.
2. Sex: The incidence and severity of atherosclerosis are more in men than in women and the changes appear a decade earlier in men (≥45 years) than in women (≥55 years).
The prevalence of atherosclerotic IHD is about three times higher in men in 4th decade than in women and the difference slowly declines with age but remains higher at all ages in men.
The lower incidence of IHD in women, especially in premenopausal age, is probably due to high levels of oestrogen and high-density lipoproteins, both of which have an anti-atherogenic influence.
3. Genetic Factors: Genetic factors play a significant role in atherogenesis.
Several hereditary derangements of lipoprotein metabolism predispose the individual to dyslipidaemia. Lipoprotein-α and apolipoprotein-C have emerged as significant causal risk factors in atherosclerosis.
4. Familial And Racial Factors: Familial hypercholesterolaemia, an autosomal codominant disorder, is characterised by elevated LDL cholesterol and normal triglycerides and the occurrence of xanthomas and premature coronary artery disease.
It occurs due to mutations in the LDL receptor gene. Familial predisposition to atherosclerosis may also be related to other risk factors like diabetes, hypertension and hyperlipoproteinaemia. Racial differences too exist; Blacks have generally less severe atherosclerosis than Whites.
3. Non-traditional Emerging Risk Factors and Biomarkers
There are several non-traditional newly emerging risk factors for which the role in etiology of atherosclerosis is yet not fully supported. Then, there are a few biomarkers which do not have a causal role in atherosclerosis but are used for coronary risk assessment.
These Factors And Biomarkers Are As Under:
1. The higher incidence of atherosclerosis in developed countries and low prevalence in underdeveloped countries suggest the role of environmental influences.
2. Use of exogenous hormones (e.g. oral contraceptives) by women or endogenous oestrogen deficiency (e.g. in post-menopausal women) has been shown to have an increased risk of developing myocardial infarction or stroke.
3. A stressful behavioural pattern, termed a ‘type A’ behaviour pattern, characterised by aggressiveness, competitive drive, ambitiousness and a sense of urgency, is associated with enhanced risk of IHD compared with ‘type B’ behaviour of the relaxed and happy-go-lucky type.
4. Hyperhomocysteinaemia is an inherited metabolic defect that leads to elevated serum homocysteine levels, a metabolite of methionine in folate and vitamin B12 metabolism. A high concentration of homocysteine is toxic to endothelium and promotes atherosclerosis.
5. Patients with homocystinuria, an uncommon inborn error of metabolism, having hypercystinaemia have also been reported to have early atherosclerosis and coronary artery
disease.
6. Prothrombotic factors and elevated fibrinogen levels cause dysregulated coagulation and favour the formation of thrombi which is the gravest complication of atherosclerosis.
Infectious burdens, particularly by multiple organisms (e.g. Chlamydia pneumonia, herpesvirus, cytomegalovirus, Helicobacter pylori, and periodontal pathogens) have been found involved in the pathogenesis of coronary atherosclerotic lesions by causing chronic inflammation.
7. The infectious agents act on the arterial wall and contribute to endothelial dysfunction, smooth muscle cell proliferation and subsequent events in the development of atheromatous plaques.
8. Excessive alcohol consumption is associated with increased progression of atherosclerosis.
However, there are some reports in the literature which suggest that moderate consumption of alcohol has a slightly beneficial effect by raising the level of HDL cholesterol.
9. Biomarkers for risk assessment Besides serum dyslipidaemia, recently several other biomarkers for coronary risk assessment have emerged.
Elevated C-reactive protein (CRP), an acute phase reactant, reflects the overall inflammatory burden due to the release of proinflammatory cytokines by adipose tissue in obese and overweight persons, and thus an indirect indicator of BMI.
Other such markers are serum homocysteine level, fibrinogen, lipoprotein-a, myeloperoxidase, and phospholipase A2. None of these is causally related to atherosclerosis but may be used as markers for coronary risk.
Pathogenesis:
As stated above, atherosclerosis is not caused by a single etiologic factor but is a multifactorial process whose exact pathogenesis is still not known. Since the time of Virchow, a number of theories have been proposed.
Insulation hypothesis: The concept hypothesised by Virchow in 1856 that atherosclerosis is a form of cellular proliferation of the intimal cells resulting from increased imbibing of lipids from the blood came to be called the ‘lipid theory.
A modified form of this theory is currently known as the ‘response to injury hypothesis’ and is nowadays the most widely accepted theory.
Encrustation hypothesis: The proposal put forth by Rokitansky in 1852 that atheroma represented a form of encrustation on the arterial wall from the components in the blood-forming thrombi composed of platelets, fibrin and leucocytes, was named ‘encrustation theory ‘thrombogenic theory’.
Currently, it is believed that encrustation or thrombosis is not the sole factor in atherogenesis but the components of thrombus (platelets, fibrin and leucocytes) have a role in atheromatous lesions, this theory has now been incorporated into the response-to-injury hypothesis mentioned above.
Monoclonal theory: This hypothesis postulated by Benditt and Benditt in 1973 is based on the neoplastic proliferation of smooth muscle cells, similar to cellular proliferation in neoplasms (e.g. in uterine leiomyoma,).
The evidence cited in support of the monoclonal hypothesis is the observation of proliferated smooth muscle cells in atheromatous plaques which have only one of the two forms of glucose-6-phosphate dehydrogenase (G6PD) isoenzymes, suggesting monoclonality in origin.
The monoclonal proliferation of smooth muscle cells in atherosclerosis may be initiated by mutation caused by exogenous chemicals (e.g. cigarette smoke), or endogenous metabolites (e.g. lipoproteins).
Currently, the origin and progression of lesion of atherosclerosis is explained by unifying all these theories into a single hypothesis called response-to-injury theory discussed below, which in essence is an inflammatory response to endothelial injury.
Response-to-Injury Theory:
This theory is most widely accepted and incorporates all aspects of previous theories on atherogenesis:
- The original response to injury theory was first described in 1976 by Ross according to which the initial event in atherogenesis was considered to be endothelial injury followed by smooth muscle cell proliferation so that the early lesions, according to this theory, consist of mainly smooth muscle cells.
- The modified response-to-injury theory implicates lipoprotein entry into the intima as the initial event followed by lipid accumulation in the macrophages (foam cells now) which according to modified theory, are believed to be the dominant cells in early lesions.
Both original and modified theories of atherogenesis assign roles to four key components in initiation, progression and complications of atherosclerosis—endothelial cells, arterial smooth muscle cells, inflammation, and dyslipidaemia
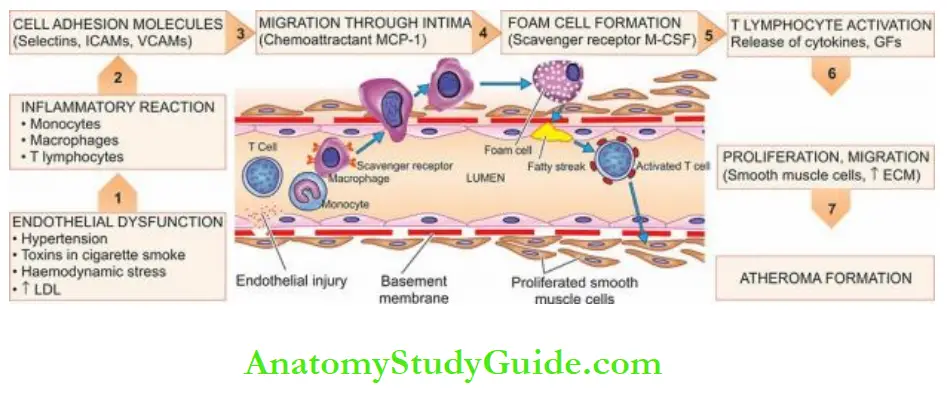
1. Endothelial injury: It has been known for many years that endothelial injury is the initial triggering event in the development of lesions of atherosclerosis.
Actual endothelial denudation is not an essential requirement, but endothelial dysfunction may initiate the sequence of events.
Numerous causes ascribed to endothelial injury in experimental animals are mechanical trauma, haemodynamic forces, immunological and chemical mechanisms, metabolic agent as chronic dyslipidaemia, homocysteine, circulating toxins from systemic infections, viruses, hypoxia,
radiation, carbon monoxide and tobacco products.
In humans, two of the major risk factors which act together to produce endothelial injury are:
Haemodynamic stress from hypertension and chronic dyslipidaemia.
The role of haemodynamic forces in causing endothelial injury is further supported by the distribution of atheromatous plaques at points of bifurcation or branching of blood vessels which are under the greatest shear stress.
2. Intimal smooth muscle cell proliferation: Endothelial injury causes adherence, aggregation and platelet release reaction at the site of exposed subendothelial connective tissue and infiltration by inflammatory cells.
The proliferation of intimal smooth muscle cells and production of the extracellular matrix are stimulated by various cytokines such as IL-1 and TNF-α released from invading monocyte macrophages and by activated platelets at the site of endothelial injury.
These cytokines lead to the local synthesis of the following growth factors having distinct roles in plaque evolution:
Platelet-derived growth factor (PDGF) and fibroblast growth factor (FGF) stimulate the proliferation and migration of smooth muscle cells from their usual location in the media into the intima.
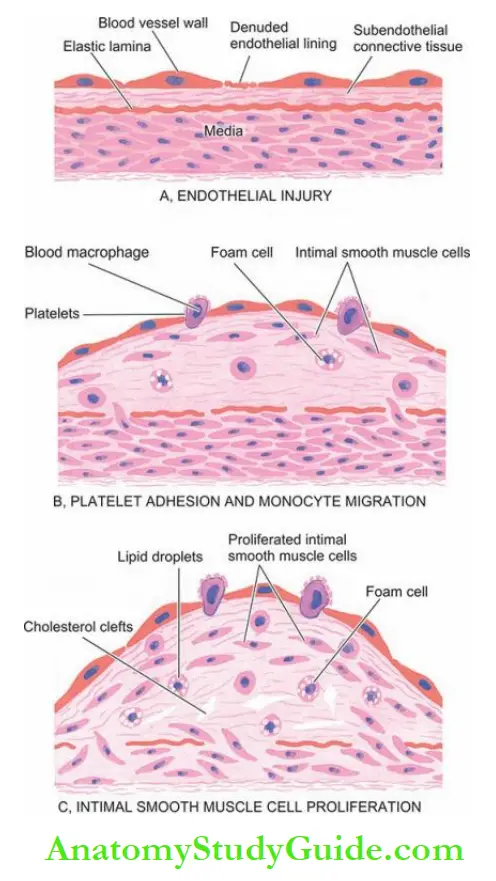
- Cytokines (transforming growth factor-β, tumour necrosis factor-β and interferon-γ) produced by activated T lymphocytes within lesions regulate the synthesis of collagen by smooth muscle cells.
- Smooth muscle cell proliferation is also facilitated by biomolecules such as nitric oxide and endothelin released from endothelial cells.
The intimal proliferation of smooth muscle cells is accompanied by the synthesis of matrix proteins—collagen, elastic fibre proteins and proteoglycans.
3. Role of inflammation: Following endothelial injury, there is an inflammatory reaction by monocytes and lymphocytes, chiefly T cells.
Plasma LDL on entry into the intima undergoes oxidation. The ‘oxidised LDL’ formed in the intima performs the following all-important functions on monocytes and endothelium
- For monocytes, Oxidised LDL acts to attract, proliferate, immobilise and activate them and is readily taken up by scavenger receptors on the monocytes to transform them to lipid-laden foam cells.
- For endothelium Oxidised LDL is cytotoxic. The lesions enlarge by attaching fibrin and cells from the blood forming thrombus that becomes a part of atheromatous plaque.
Death of foam cells by apoptosis releases lipids to form the lipid core of plaque.
4. Role of dyslipidaemia: As stated already, chronic dyslipidaemia in itself may initiate endothelial injury and dysfunction by causing increased permeability.
In particular, hypercholesterolaemia with an increased serum concentration of LDL promotes the formation of foam cells, while a high serum concentration of HDL has an anti-atherogenic effect.
Unifying Role Of All Components:
Currently, inflammation occupies a central role in the development of lesions in atherosclerosis:
1. Endothelial dysfunction by major atherogenic risk factors initiates the process.
Endothelial dysfunction may be by mechanical injury (e.g. haemodynamic injury in hypertension) or by chemical (e.g. dyslipidaemia, diabetes, smoking etc), or immunological insult.
2. This triggers a cascade of inflammatory reactions that involve monocytes, macrophages, T lymphocytes and smooth muscle cells.
3. Many injured endothelial cells express cell adhesion molecules on their surface (selectins, ICAMs, VCAMs) which act as receptors for integrins on the surface of monocytes and T cells.
4. Next, monocytes migrate through the intima by chemoattractant molecules, monocyte chemoattractant protein-1 (MCP-1).
5. Once inside the intima, monocytes become macrophages and express scavenger receptors, on their surface for LDL called macrophage-colony stimulating factor (M-CSF).
M-CSF plays multiple roles for macrophages: ingestion of lipids, multiplication and differentiation of monocytes into macrophage foam cells. This leads to initial lesions of atherosclerosis, and fatty streaks.
6. Fatty streak lesions activate T lymphocytes which secrete several cytokines and growth factors.
These lead to the proliferation of smooth muscle cells and fibroblasts and the production of extracellular matrix. Repeated cycles of the same process lead to a fully-developed atheroma.
Morphologic Features:
Early lesions in the form of diffuse intimal thickening, fatty streaks and gelatinous lesions are often the forerunners in the evolution of atherosclerotic lesions.
However, the clinical disease states due to luminal narrowing in atherosclerosis are caused by fully developed atheromatous plaques and complicated plaques.
1. Fatty Streaks And Dots:
Fatty streaks and dots on the intima by themselves are harmless but may be the precursor lesions of atheromatous plaques. They are seen in all races of the world and begin to appear in the first year of life.
However, they are uncommon in older persons and are probably absorbed. They are especially prominent in the aorta and other major arteries, more often on the posterior wall than the anterior wall.
Grossly, the lesions may appear flat or slightly elevated and yellow.
They may be either in the form of small, multiple dots, about 1 mm in size, or in the form of elongated, beaded streaks.
Microscopically, fatty streaks lying under the endothelium are composed of closely-packed foam cells, lipid-containing elongated smooth muscle cells and a few lymphoid cells.
Small amounts of extracellular lipids, collagen and proteoglycans are also present.
2. Gelatinous Lesions:
Gelatinous lesions develop in the intima of the aorta and other major arteries in the first few months of life. Like fatty streaks, they may also be precursors of plaques. They are round or oval, circumscribed grey elevations, about 1 cm in diameter.
Microscopically, gelatinous lesions are foci of increased ground substance in the intima with thinned overlying endothelium.
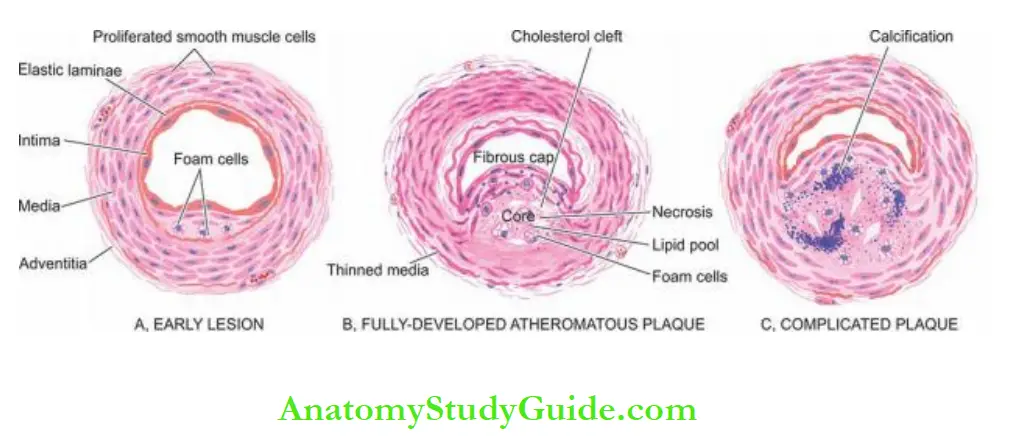
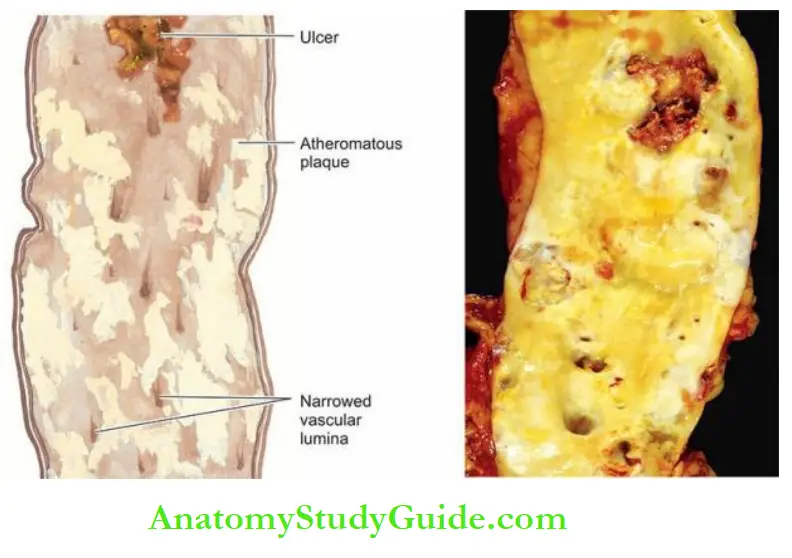
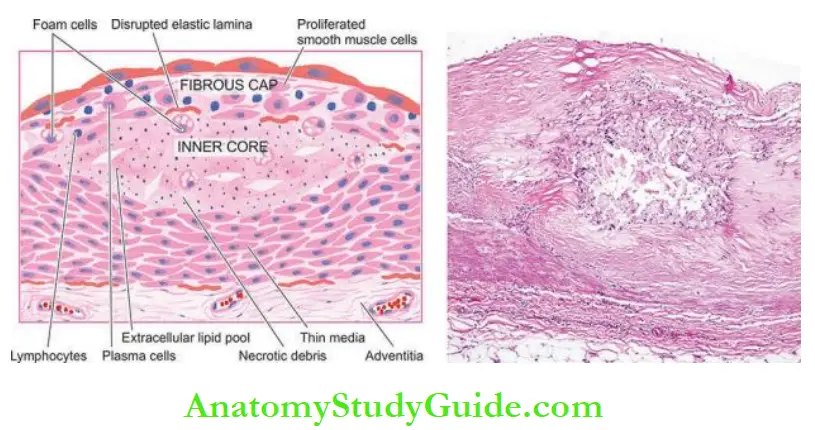
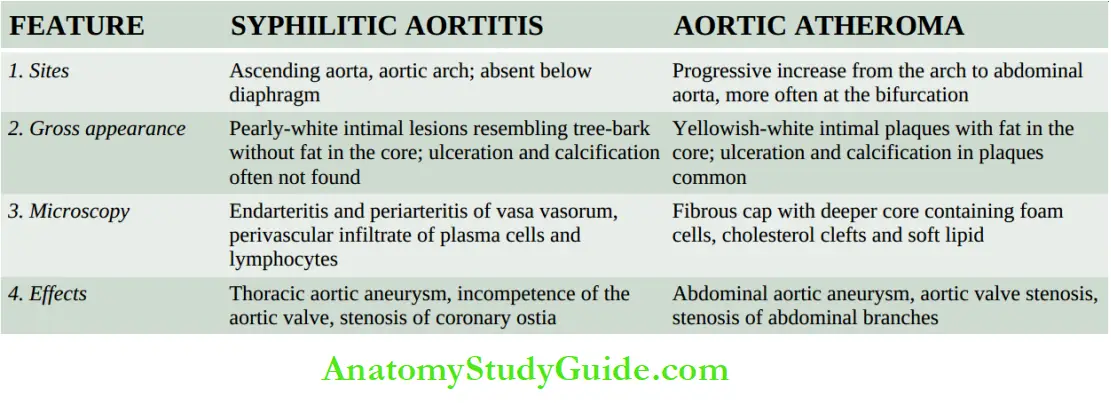
3. Atheromatous Plaques: A fully developed atherosclerotic lesion is called atheromatous plaque, also called fibrous plaque, fibrofatty plaque or atheroma.
Unlike fatty streaks, atheromatous plaques are selective in different geographic locations and races and are seen in advanced age.
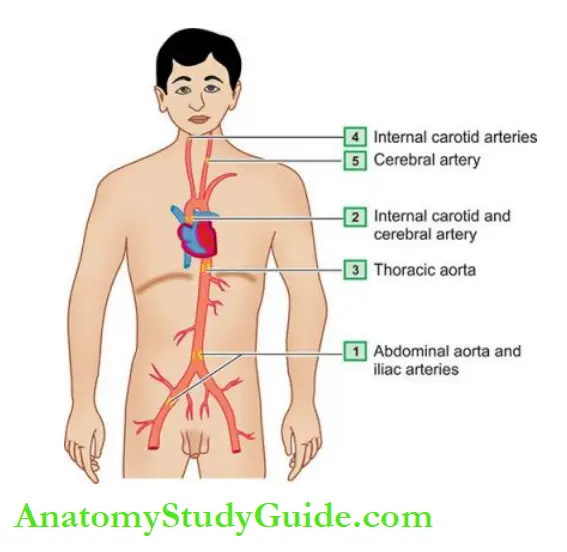
These lesions may develop from the progression of early lesions of atherosclerosis just described. Most often and most severely affected is the abdominal aorta, though smaller lesions may be seen in descending thoracic aorta and aortic arch.
The major branches of the aorta around the ostia are often severely involved, especially the iliac, femoral, carotid, coronary, and cerebral arteries.
Grossly, atheromatous plaques are white to yellowish-white lesions, varying in diameter from 1-2 cm and raised on the surface by a few millimetres to a centimetre in thickness.
The cut section of the plaque reveals the luminal surface as a firm, white fibrous cap and a central core composed of yellow to yellow-white, soft, porridge-like material and hence the name atheroma.
Microscopically, the appearance of plaque varies depending on the age of the lesion.
However, the following features are invariably present:
- The superficial luminal part of the fibrous cap is covered by endothelium and is composed of smooth muscle cells, dense connective tissue and an extracellular matrix containing proteoglycans and collagen.
- The cellular area under the fibrous cap is comprised of a mixture of macrophages, foam cells, lymphocytes and a few smooth muscle cells which may contain lipids.
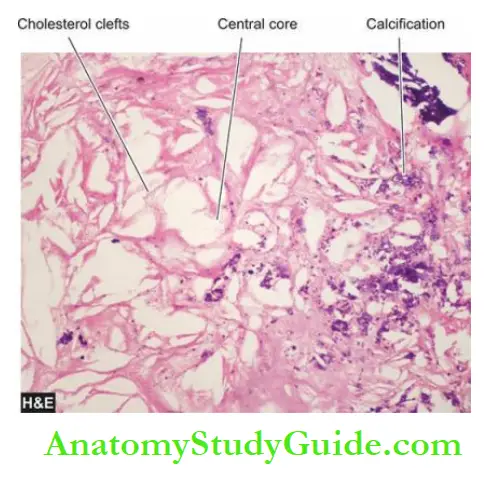
- The deeper central soft core consists of extracellular lipid material, cholesterol clefts, fibrin, necrotic debris and lipid-laden foam cells.
- In older and more advanced lesions, the collagen in the fibrous cap may be dense and hyalinised, smooth muscle cells may be atrophic and foam cells are fewer.
4. Complicated Plaques:
Various pathologic changes that occur in fully-developed atheromatous plaques are called complicated lesions.
These account for the most serious harmful effects of atherosclerosis and even death.
These changes include calcification, ulceration, thrombosis, haemorrhage and aneurysmal dilatation.
It is not uncommon to see more than one form of complication in a plaque.
1. Calcification: Calcification occurs more commonly in advanced atheromatous plaques, especially in the aorta and coronaries. The diseased intima cracks like an eggshell when the vessel is incised and opened.
Microscopically, the calcium salts are deposited in the vicinity of the necrotic area and in the soft lipid pool deep in the thickened intima.
This form of atherosclerotic intimal calcification differs from Mönckeberg’smedial calcific arteriosclerosis that affects only the tunica media.
2. Ulceration: The layers covering the soft pultaceous material of atheroma may ulcerate as a result of haemodynamic forces or mechanical trauma.
This results in the discharge of emboli composed of lipid material and debris into the bloodstream, leaving a shallow, ragged ulcer with yellow lipid debris in the base of the ulcer.
Occasionally, atheromatous plaque in a coronary artery may suddenly rupture into the arterial lumen forcibly and cause thromboembolic occlusion.
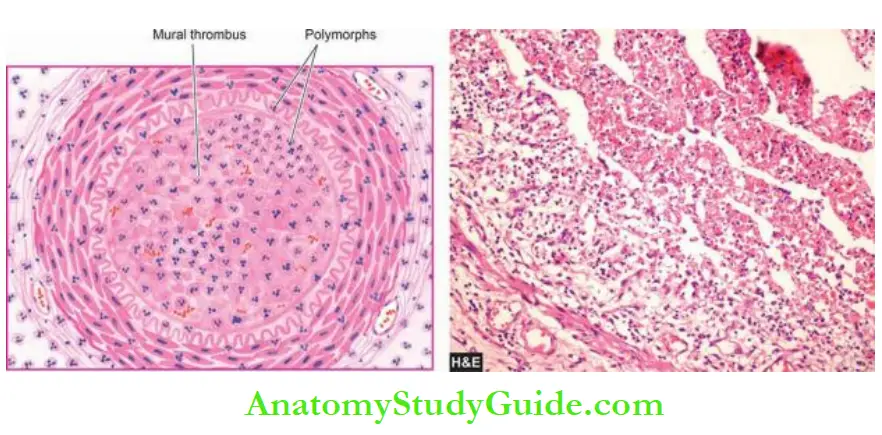
3. Thrombosis: The ulcerated plaque and the areas of endothelial damage are vulnerable sites for the formation of superimposed thrombi.
These thrombi may get dislodged to become emboli and lodge elsewhere in the circulation or may get organised and incorporated into the arterial wall as mural thrombi.
Mural thrombi may become occlusive thrombi which may subsequently recanalise.
4. Haemorrhage: Intimal haemorrhage may occur in an atheromatous plaque either from the blood in the vascular lumen through an ulcerated plaque or from rupture of thin-walled capillaries that vascularise the atheroma from adventitial vasa vasorum.
Haemorrhage is particularly a common complication in coronary arteries. The haematoma formed at the site contains numerous haemosiderin-laden macrophages.
5. Aneurysm formation: Though atherosclerosis is primarily an intimal disease, advanced lesions are associated with secondary changes in the media and adventitia.
The changes in media include atrophy and thinning of the media and fragmentation of internal elastic lamina.
The adventitia undergoes fibrosis and some inflammatory changes.
These changes cause weakening in the arterial wall resulting in aneurysmal dilatation.
Clinical Effects
The clinical effects of atherosclerosis depend upon the size and type of arteries affected.
In general, the clinical effects result from the following:
- The slow luminal narrowing causes ischaemia and atrophy.
- Sudden luminal occlusion causing infarction necrosis.
- Propagation of plaque by the formation of thrombi and emboli.
- Formation of aneurysmal dilatation and eventual rupture.
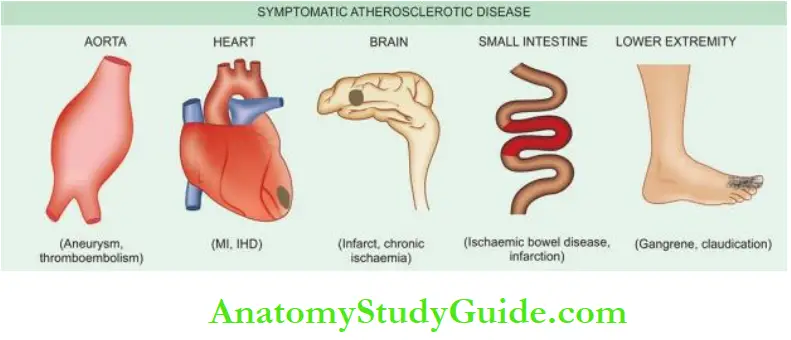
Large arteries affected most often are the aorta, renal, mesenteric and carotids, whereas the medium- and small-sized arteries frequently involved are the coronaries, cerebral and arteries of the lower limbs.
Accordingly, the symptomatic atherosclerotic disease involves most often the heart, brain, kidneys, small intestine and lower extremities.
The effects pertaining to these organs are described in relevant chapters later while the major effects are listed below:
- Heart—Myocardial infarction, ischaemic heart disease.
- Brain—Chronic ischaemic brain damage, cerebral infarction and stroke.
- Aorta—Aneurysm formation, thrombosis and embolisation to other organs.
- Small intestine—Ischaemic bowel disease, infarction.
- Lower extremities—Intermittent claudication, gangrene.
Atherosclerosis:
- Atherosclerosis is the thickening and hardening of large and medium-sized muscular arteries, primarily due to the involvement of tunica intima and is characterised by fibrofatty plaques or atheromas.
- Major risk factors modifiable by lifestyle and/or therapy include dyslipidaemias, hypertension, diabetes mellitus, smoking and lifestyle risk factors (atherogenic diet, obesity and physical inactivity).
- Constitutional non-modifiable risk factors are advancing age, male sex, genetic influences, familial predisposition and white race.
- Non-traditional emerging risk factors are: environment, exogenous oestrogen, stressful behaviour, raised homocysteine, homocystinuria, prothrombotic and proinflammatory factors and excessive alcohol consumption
- Risk assessment biomarkers are CRP, lipoprotein-a, fibrinogen etc.
- Atherogenesis is explained commonly by the reaction to injury hypothesis that involves:
- endothelial injury, intimal smooth muscle cell proliferation, circulating monocytes, dyslipidaemia and thrombosis.
- The lesions of atherosclerosis begin with fatty streaks and gelatinous lesions.
- Full-blown atheromatous lesions or fibrofatty plaques have a superficial cap and cellular or soft centre.
- Complicated atheromas may have dystrophic calcification, ulceration, thrombosis, haemorrhage and aneurysm formation.
- Major clinical effects of atherosclerosis are on the heart (coronary artery disease), brain (stroke), aorta (aneurysmal dilatation), intestine (ischaemia) and lower extremities
(gangrene)
Vasculitis
Arteritis, angiitis and vasculitis are the common terms used for inflammatory involvement of any size, type and location of vessel wall—artery, arterioles, venules and capillaries.
The term vasculitis syndrome is used for the pathologic involvement of vessels that produces a heterogeneous and overlapping complex of clinical features.
Vasculitis is variously classified based on pathophysiology or aetiology:
Pathophysiologic classification: Based on pathophysiology, vasculitis is divided into primary and secondary.
- When the involvement of a vessel is the sole or primary manifestation, it is termed primary vasculitis.
- When the vessel is secondarily involved as a part of a disease, it is called secondary vasculitis.
Etiologic classification: Based on causes, vasculitis may occur following the invasion of the vessel by microbial agents (infectious vasculitis) or may be induced by non-infectious injuries such as chemical, mechanical, immunologic and radiation injury (non-infectious vasculitis).
Clinicopathologically, the non-infectious group is more significant than the infectious type.
A classification of vasculitis based on aetiology Some common forms are discussed below.
1. Infectious Arteritis
- Endarteritis obliterans
- Syphilitic arteritis
- Non-specific infective arteritis
2. Non-Infectious Arteritis
- Polyarteritis nodosa (PAN)
- Hypersensitivity (allergic, leukocytoclastic) vasculitis
- Wegener’s granulomatosis
- Temporal (giant cell) arteritis
- Takayasu arteritis (pulseless disease)
- Kawasaki disease
- Buerger’s disease (thromboangiitis obliterans)
1. Infectious Arteritis
Direct invasion of the artery by infectious agents, especially bacteria and fungi, causes infectious arteritis.
It may be found in the vicinity of an infected focus like in tuberculosis, pneumonia, abscesses, etc. or less frequently may arise from the haematogenous spread of infection such as in infective endocarditis, septicaemia etc.
Endarteritis Obliterans:
Endarteritis obliterans is not a disease entity but a pathologic designation used for the non-specific inflammatory response of arteries and arterioles to a variety of irritants.
It is commonly seen close to the lesions of peptic ulcers of the stomach and duodenum, tuberculous and chronic abscesses in the lungs, chronic cutaneous ulcers, chronic meningitis, and in post-partum and post-menopausal uterine arteries.
Grossly, the affected vessels may appear unaltered externally but on cross-section show obliteration of their lumina.
Microscopically, the obliteration of the lumen is due to the concentric and symmetric proliferation of cellular fibrous tissue in the intima.
Though the condition has the suffix—itis attached to it, there is minimal or no inflammatory cell infiltrate.
Syphilitic Arteritis:
Syphilitic or luetic vascular involvement occurs in all stages of syphilis but is more prominent in the tertiary stage.
The changes that are found in syphilitic arteritis are seen within the arterial tissue (syphilitic endarteritis) and in the periarterial tissues (syphilitic periarteritis).
Manifestations of the disease are particularly prominent at two sites—the aorta and the cerebral arteries.
Syphilitic Aortitis:
Syphilitic involvement of the ascending aorta and the aortic arch is the commonest manifestation of cardiovascular syphilis. It occurs in about 80% of cases of tertiary syphilis.
Preferential involvement of the arch of the aorta may be due to the involvement of mediastinal lymph nodes in secondary syphilis through which the treponemes spread to the lymphatics around the aortic arch.
The lesions diminish in severity in descending thoracic aorta and disappear completely at the level of the diaphragm.
Grossly, the affected part of the aorta may be dilated, and its wall is somewhat thickened and adherent to the neighbouring mediastinal structures.
Longitudinally opened vessels show intimal surfaces studded with pearly-white thickenings, varying from a few millimetres to a centimetre in diameter.
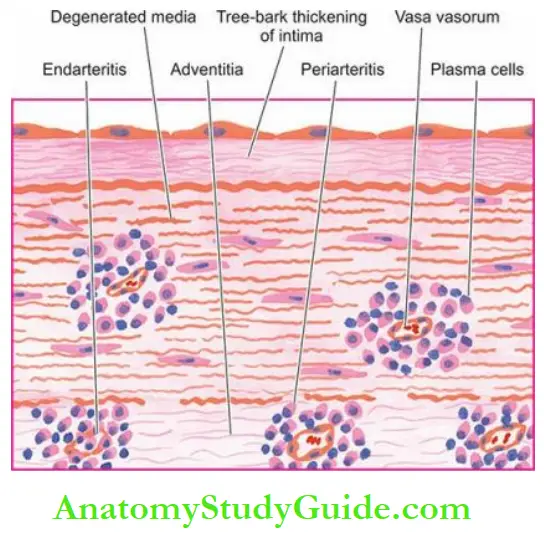
These lesions are separated by wrinkled normal intima, giving it a characteristic tree-bark appearance.
The cut section of the lesion shows a more firm and fibrous appearance than the atheromatous plaques. However, superimposed atherosclerotic lesions may be present.
Microscopically, the conspicuous features are as under:
- Endarteritis and periarteritis of the vasa vasorum located in the media and adventitia.
- Perivascular accumulation of plasma cells, lymphocytes and macrophages that may form miliary gummas which undergo necrosis and are replaced by scar tissue.
- Intimal thickenings consist of dense avascular collagen that may undergo hyalinisation and calcification.
The effects of syphilitic aortitis may vary from trivial to catastrophic. These are as follows:
- An aortic aneurysm may result from damage to the aortic wall.
- Aortic valvular incompetence used to be considered an important sequela of syphilis but nowadays rheumatic disease is considered its more important cause.
- The aortic incompetence results from the spread of the syphilitic process to the aortic valve ring.
- Stenosis of coronary ostia is seen in about 20% of cases of syphilitic aortitis and may lead to progressive myocardial fibrosis, angina pectoris and sudden death.
The features distinguishing syphilitic aortitis from aortic atheroma.
Cerebral Syphilitic Arteritis (Heubner’s Arteritis)
Syphilitic involvement of small and medium-sized cerebral arteries occurs during tertiary syphilis. The changes may accompany syphilitic meningitis.
Grossly, the cerebral vessels are white, rigid and thick-walled.
Microscopically, changes in endarteritis and periarteritis similar to those seen in syphilitic aortitis are found.
There is atrophy of muscle in the media and replacement by fibrosis. This results in ischaemic atrophy of the brain.
Non-Specific Infective Arteritis:
Various forms of invasions of the artery by bacteria, fungi, parasites or viruses, either directly or by haematogenous route, cause non-syphilitic infective arteritis.
Microscopically, the inflammatory infiltrate is present in the vessel wall.
The vascular lumen may get occluded by thrombi and result in ischaemic necrosis of the affected tissue.
2. Non-Infectious Arteritis:
This group consists of most of the important forms of vasculitis, more often affecting arterioles, venules and capillaries, and hence also termed small vessel vasculitis.
Their exact etiology is not known but available evidence suggests that many of them have immunologic origin.
Serums from many patients with vasculitis of immunologic origin show the presence of the following immunologic features:
1. Anti-neutrophil cytoplasmic antibodies (ANCAs): Patients with immunologic vasculitis have autoantibodies in their serum against the cytoplasmic antigens of the neutrophils, macrophages and endothelial cells; these are called ANCAs.
Neutrophil immunofluorescence is used to demonstrate their presence, of which two distinct patterns of ANCAs are seen:
- Cytoplasmic ANCA (c-ANCA)pattern is specific for proteinase-3 (PR-3) a constituent of neutrophilic granules; this is seen in cases with active Wegener’s granulomatosis.
- Perinuclear ANCA (p-ANCA)pattern is specific for myeloperoxidase enzyme; this is noted in patients with microscopic polyarteritis nodosa and primary glomerular disease.
2. Anti-endothelial cell antibodies (AECAs): These antibodies are demonstrable in cases of SLE, Kawasaki disease and Buerger’s disease.
3. Pauci-immune vasculitis: While most cases of immunologic vasculitis have immune complex deposits in the vessel wall, there are some cases which do not have such immune deposits and are termed as cases of pauci-immune vasculitis (similar to pauci-immune glomerulonephritis. Pathogenesis of lesions in these cases is explained by other mechanisms.
Polyarteritis Nodosa:
Polyarteritis nodosa (PAN) is a necrotising vasculitis involving small and medium-sized muscular arteries of multiple organs and tissues.
‘Polyarteritis’ is the preferred nomenclature over ‘periarteritis’ because inflammatory involvement occurs in all the layers of the vessel wall.
The disease occurs more commonly in adult males than females.
The most commonly affected organs, in descending order of frequency of involvement, are the kidneys, heart, liver, gastrointestinal tract, muscle, pancreas, testes, nervous system and skin.
The syndrome of PAN presents with varied symptoms pertaining to different organs.
However, some usual clinical features are fever, malaise, weakness, weight loss, renal manifestations (albuminuria, haematuria and renal failure), vascular lesions in the alimentary tract (abdominal pain and melaena), peripheral neuritis and hypertension.
The condition is believed to result from the deposition of immune complexes and tumour-related antigens.
Grossly, the lesions of PAN involve segments of vessels, especially at the bifurcations and branchings, as tiny beaded nodules.
Microscopically, there are 3 sequential stages in the evolution of lesions in PAN
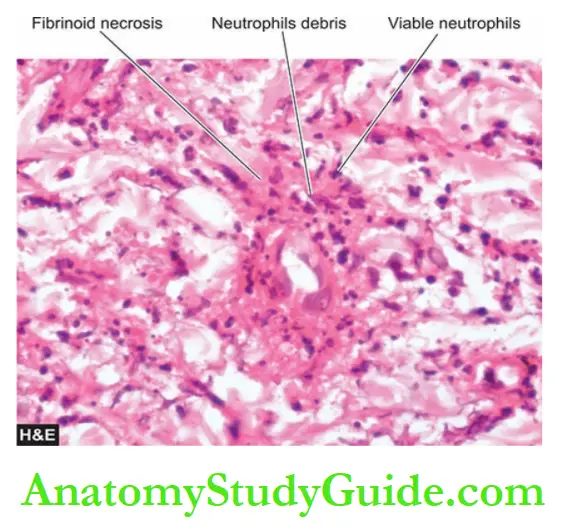
1. Acute stage—There is fibrinoid necrosis in the centre of the nodule located in the media.
An acute inflammatory response develops around the focus of fibrinoid necrosis.
The inflammatory infiltrate is present in the entire circumference of the affected vessel (periarteritis) and consists chiefly of neutrophils and eosinophils, and some mononuclear cells.
The lumen may show thrombi and the weakened wall may be the site of aneurysm formation.
2. Healing stage—This is characterised by marked fibroblastic proliferation producing firm nodularity.
The inflammatory infiltrate now consists mainly of lymphocytes, plasma cells and macrophages.
3. Healed stage—In this stage, the affected arterial wall is markedly thickened due to dense fibrosis.
The internal elastic lamina is fragmented or lost. The healed stage may contain haemosiderin-laden macrophages and an organised thrombus.
However, it may be mentioned here that various stages of the disease may be seen in different vessels and even within the same vessel.
Hypersensitivity Vasculitis:
Hypersensitivity vasculitis, also called allergic or leukocytoclastic vasculitis or microscopic polyarteritis, is a group of clinical syndromes differing from PAN in having inflammatory involvement of venules, capillaries and arterioles.
The tissues and organs most commonly involved are the skin, mucous membranes, lungs, brain, heart, gastrointestinal tract, kidneys and muscle.
The condition results from an immunologic response to an identifiable antigen that may be bacteria (e.g. streptococci, staphylococci, mycobacteria), viruses (e.g. hepatitis B virus, influenza virus, CMV), malarial parasites, certain drugs and chemicals.
Hypersensitivity vasculitis includes clinicopathologic entities such as serum sickness, Henoch-Schonlein purpura, mixed cryoglobulinaemia, vasculitis associated with malignancy, and vasculitis associated with connective tissue diseases like rheumatoid arthritis and SLE.
Microscopically, the lesions characteristically involve the smallest vessels, sparing medium-sized and larger arteries.
Two histologic forms are described:
- Leukocytoclastic vasculitis, characterised by fibrinoid necrosis with neutrophilic infiltrate in the vessel wall. Many of the neutrophils are fragmented. This form is found in vasculitis caused by deposits of immune complexes.
- Lymphocytic vasculitis, in which the involved vessel shows predominant infiltration by lymphocytes. This type is seen in vascular injury due to delayed hypersensitivity or cellular immune reactions.
Wegener’s Granulomatosis:
Wegener’s granulomatosis is another form of necrotising vasculitis characterised by a clinicopathologic triad consisting of the following:
- Acute necrotising granulomas of the upper and lower respiratory tracts involving the nose, sinuses and lungs,
- focal necrotising vasculitis, particularly of the lungs and upper airways, and
- focal or diffuse necrotising glomerulonephritis. A limited form of Wegener’s granulomatosis is the same condition without renal involvement.
As with PAN, the condition is more common in adult males and involves multiple organs and tissues.
The most commonly involved organs are the lungs, paranasal sinuses, nasopharynx and kidneys. Other involved organs are joints, skin, eyes, ears, heart and nervous system.
Accordingly, clinical features are variable. Typical features include pneumonitis with bilateral infiltrates in the lungs, chronic sinusitis, and nasopharyngeal ulcerations. and renal disease.
The etiology is not known but possibly the lesions occur due to the presence of circulating immune complexes.
This is supported by the observation of subepithelial immunoglobulin deposits on the glomerular basement membrane and induction of remission by immunosuppressive therapy.
The serum of these patients shows c-ANCA positivity.
The disseminated form of Wegener’s granulomatosis differs from a related entity, idiopathic lethal midline granuloma, in the sense that the latter condition is a highly destructive
and progressively necrotic disease of the upper airways.
Histologically, the characteristic feature of Wegener’s granulomatosis is the presence of necrotising granulomatous inflammation of the tissues and necrotising vasculitis with or without granulomas:
- The granulomas consist of fibrinoid necrosis with extensive infiltration by neutrophils, mononuclear cells, epithelioid cells, multinucleate giant cells and fibroblastic proliferation.
- The necrotising vasculitis may be segmental or circumferential.
- The renal lesions are those of focal or diffuse necrotising glomerulonephritis.
Temporal (Giant Cell) Arteritis
This is a form of granulomatous inflammation of medium-sized and large arteries.
Preferential sites of involvement are the cranial arteries, especially the temporal, hence the name.
However, the aorta and other major arteries like common carotid, axillary, brachial, femoral and mesenteric arteries are also involved, and therefore, it is preferable to call the entity ‘giant cell arteritis’.
The patients are generally over the age of 70 years with slight female preponderance.
The usual clinical manifestations are headache and blindness if the ophthalmic artery is involved.
An association with polymyalgia rheumatica has been observed.
The cause of the condition remains unknown though there is the suggestion of T cell-mediated immunologic reaction to some
component of the arterial wall, especially against the damaged internal elastic lamina.
Biopsy of the affected artery is not only of diagnostic value but also relieves the patient of painful symptoms.
Grossly, the affected artery is thickened, and cord-like and the lumen is usually reduced to a narrow slit.
Histologically, the features include the following:
- There is a chronic granulomatous reaction, usually around the internal elastic lamina and typically involves the entire circumference of the vessel.
- Giant cells of foreign bodies or Langhans’ type are found in two-thirds of cases.
- The internal elastic lamina is often fragmented.
- There is eccentric or concentric intimal cellular proliferation causing marked narrowing of the lumen.
- The narrowed lumen may contain a thrombus.
- Occasionally, only nonspecific inflammatory cell infiltrate consisting of neutrophils, lymphocytes and eosinophils is found throughout the arterial wall.
Takayasu Arteritis (Pulseless Disease):
This is a form of granulomatous vasculitis affecting chiefly the aorta and its major branches and hence is also referred to as an aortic arch syndrome.
The disease affects chiefly young women and is typically characterised by the absence of a pulse in both arms and the presence of ocular manifestations.
Other features referable to ischaemic effects from thrombotic occlusion of vessels include myocardial infarction, congestive heart failure and neurologic deficits.
The etiology of Takayasu arteritis is not known but the autoimmune reaction to aortic tissue has been suggested as the possible cause.
Grossly, the aortic wall is irregularly thickened and intima wrinkled.
The branches of major arteries coming off the aortic arch have obliterated lumina.
Histologically, the features are as under:
- There is a severe mononuclear inflammatory infiltrate involving the full thickness of the affected vessel wall.
- The inflammatory changes are more severe in the adventitia and media and there is perivascular infiltration of the vasa vasorum.
- Granulomatous changes in the media with central necrosis and Langhans’ giant cells are found in many cases.
- Advanced lesions show extensive fibrosis of the media and adventitia causing thickening in the vessel wall.
Kawasaki Disease:
Also known by the more descriptive name of ‘mucocutaneous lymph node syndrome’, it is an acute and subacute illness affecting mainly young children and infants.
Kawasaki disease is a febrile illness with mucocutaneous symptoms like erosions of oral mucosa and conjunctiva, skin rash and lymphadenopathy.
The aetiology is unknown; possible causes considered are infectious, genetic, toxic and immunological.
The most characteristic finding is the presence of multiple aneurysms of the coronaries detected by angiography during life or observed at autopsy.
Other vessels that may be involved are renal, mesenteric, hepatic and pancreatic arteries.
Histologically, the picture is of pan arteritis resembling PAN, characterised by necrosis and inflammation of the entire thickness of the vessel wall.
Therefore, some consider Kawasaki’s disease as an infantile form of PAN.
Buerger’s Disease (Thromboangiitis Obliterans):
Buerger’s disease is a specific disease entity affecting chiefly small and medium-sized arteries and veins of the extremities and characterised by acute and chronic occlusive inflammatory involvement.
The disease affects chiefly men under the age of 35 years who are heavy cigarette smokers.
It is more prevalent in Asians and persons of East European descent.
The symptom complex consists of intermittent claudication due to ischaemia manifested by intense pain affecting the limbs, more commonly the legs.
Eventually, gangrene of the affected extremities occurs requiring amputation.
Etiopathogenesis:
The following possible mechanisms have been suggested:
- There is a consistent association with heavy cigarette smoking. This has led to the hypothesis that tobacco products cause either direct endothelial damage leading to hypercoagulability and thrombosis, or is a result of hypersensitivity to tobacco products. In support is the demonstration of anti-endothelial cell antibodies (AECAs).
- Genetic factors play a role as the disease has familial occurrence and has an HLA association. An increased prevalence is seen in individuals with HLA-A9 and HLA-B5 antigens.
Grossly, the lesions are typically segmental affecting small and medium-sized arteries, especially of the lower extremities.
The involvement of the arteries is often accompanied by the involvement of adjacent veins and nerves.
Fibrous tissue cuff generally surrounds these three structures. Mural thrombi are frequently present in the vessels.
Microscopically, the following changes are seen in different stages of the disease:
In the early stage, there is infiltration by polymorphs in all the layers of vessels and there is an invariable presence of mural or occlusive thrombosis of the lumen.
The appearance differs from atherosclerosis in having microabscesses in the thrombi, the proliferation of endothelial cells, lack of lipid aggregates and the presence of intact internal elastic lamina.
In the advanced stage, the cellular infiltrate is predominantly mononuclear and may contain an occasional epithelioid cell granuloma with Langhans’ giant cells.
The thrombi undergo organisation and recanalisation. In more chronic cases, marked fibrosis of the media is present.
Miscellaneous Hypersensitivity Vasculitis:
Various connective tissue diseases (e.g. rheumatoid arthritis, ankylosing spondylitis and SLE), rheumatic fever, certain malignancies and Henoch-Schonlein purpura are associated with vasculitis.
The type of vasculitis is generally hypersensitivity or allergic angiitis as already explained but sometimes may resemble PAN.
- Rheumatoid vasculitis affects chiefly the small and medium-sized arteries of multiple visceral organs in patients who have rheumatoid nodules of long duration. Vasculitis in SLE affects mainly the small arteries of the skin.
- Rheumatic vasculitis involves the aorta, carotid and coronary arteries and the visceral vessels. Usually, fibrinoid change and perivascular inflammation are seen rather than typical Aschoff nodules.
Raynaud’s Disease And Raynaud’s Phenomenon:
Raynaud’s disease is not a vasculitis but is a functional vasospastic disorder affecting chiefly small arteries and arterioles of the extremities, occurring in otherwise young healthy females but is discussed here due to clinical features overlapping with Buerger’s disease.
The disease affects most commonly the fingers and hands. The ischaemic effect is provoked primarily by cold but other stimuli such as emotions, trauma, hormones and drugs also play a role.
Clinically, the affected digits show pallor, followed by cyanosis, and then redness, corresponding to arterial ischaemia, venostasis and hyperaemia respectively.
Long-standing cases may develop ulceration and necrosis of digits but the occurrence of true gangrene is rare.
The cause of the disease is unknown but probably occurs due to vasoconstriction mediated by autonomic stimulation of the affected vessels.
Though usually no pathologic changes are observed in the affected vessels, long-standing cases may show endothelial proliferation and intimal thickening.
Primary Raynaud’s phenomenon differs from Raynaud’s disease in having an underlying cause e.g.
secondary to atherosclerosis, connective tissue diseases like scleroderma and SLE, Buerger’s disease, multiple myeloma, pulmonary hypertension and ingestion of an ergot group of drugs.
Raynaud’s phenomenon like Raynaud’s disease, also shows cold sensitivity but differs from the latter in having structural abnormalities in the affected vessels.
These changes include segmental inflammation and fibrinoid change in the walls of capillaries.
Vasculitis:
- Arteritis, angiitis and vasculitis are the terms used for inflammatory involvement of the vessel wall of different types, sizes and locations i.e. arteries, arterioles, venules or capillaries.
- Vasculitis syndrome is a complex of clinical features in various forms of vasculitis—primary or secondary.
- It may be caused by infectious agents or induced by non-infectious injuries; the latter is more important and more common.
- Infectious vasculitis may be nonspecific or syphilitic.
- Non-infectious vasculitis is often of immunologic origin and may be positive for serum antibodies: ANCA (c-ANCA or p-ANCA) or AECA. Common examples of non-infectious vasculitis are polyarteritis nodosa, hypersensitivity vasculitis, Wegener’s granulomatosis, temporal arteritis, Takayasu arteritis, Kawasaki disease and Buerger’s disease.
Raynaud’s disease is a vasospastic disorder in young women and not a vasculitis.
Aneurysms
Definition And Classification:
An aneurysm is defined as a permanent abnormal dilatation of a blood vessel occurring due to congenital or acquired weakening or destruction of the vessel wall.
Most commonly, aneurysms involve large elastic arteries, especially the aorta and its major branches.
Aneurysms can cause various ill effects such as thrombosis and thromboembolism, alteration in the flow of blood, rupture of the vessel and compression of neighbouring structures.
Aneurysms can be classified on the basis of various features:
1. Depending upon the composition of the wall
- A true aneurysm is composed of all the layers of a normal vessel wall.
- False aneurysms have fibrous walls and occur often from trauma to the vessel.
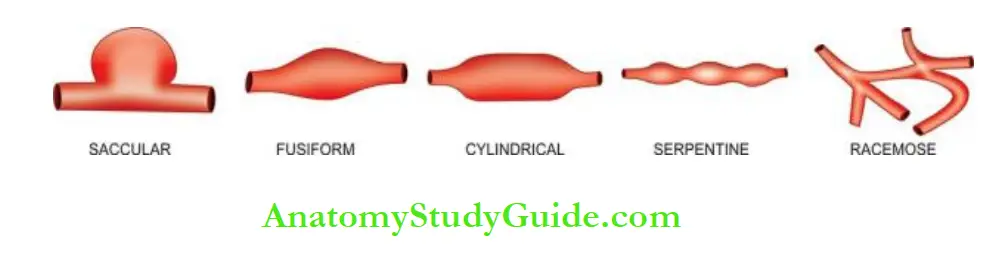
2. Depending upon the shape These are as under:
- Saccular has large spherical outpouching.
- Fusiform has slow spindle-shaped dilatation.
- Cylindrical with a continuous parallel dilatation.
- Serpentine or varicose which has tortuous dilatation of the vessel.
- Racemose or circoid has a mass of intercommunicating small arteries and veins.
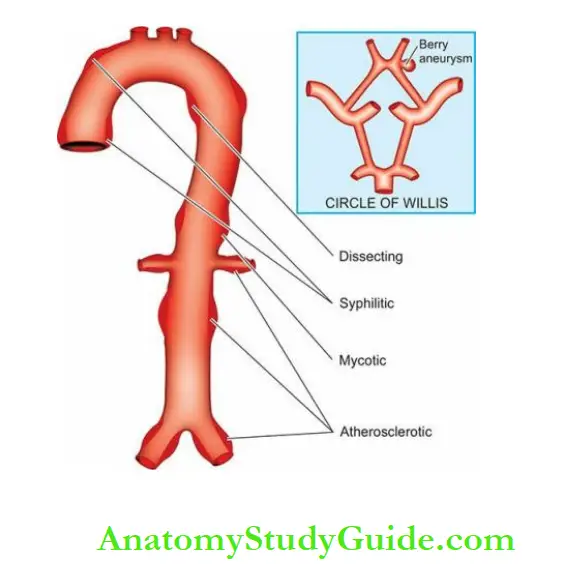
3. Based on pathogenetic mechanisms
This classification is followed more often:
- Atherosclerotic (arteriosclerotic) aneurysms are the most common type.
- Syphilitic (luetic) aneurysms are found in the tertiary stage of syphilis.
- Dissecting aneurysms (Dissecting haematoma) in which the blood enters the separated or dissected wall of the vessel.
- Mycotic aneurysms result from the weakening of the arterial wall by microbial infection.
- Berry aneurysms are small dilatations, especially affecting the circle of Willis in the base of the brain.
Out of these, three common types of aortic aneurysms—atherosclerotic, syphilitic and dissecting, are described below, followed by brief comments on fibromuscular dysplasia.
Atherosclerotic Aneurysms:
Atherosclerotic aneurysms are the most common form of aortic aneurysms.
They are seen more commonly in males and the frequency increases after the age of 50 years when the incidence of complicated lesions of advanced atherosclerosis is higher.
They are most common in the abdominal aorta, so much so that all forms of aneurysms of the abdominal aorta (fusiform, cylindrical and saccular) should be considered atherosclerotic until proven otherwise.
Other locations include the thoracic aorta (essentially the ascending part and arch of the aorta), iliac arteries and other large systemic arteries.
Pathogenesis:
Obviously, severe atherosclerotic lesions are the basic problem which causes thinning and destruction of the medial elastic tissue resulting in atrophy and weakening of the
wall.
Since atherosclerotic lesions are most common and severe in the abdominal aorta, atherosclerotic aneurysms occur most frequently here.
In the thoracic aorta, besides atherosclerotic lesions, medial degeneration is another additional factor implicated in pathogenesis.
Morphologic Features:
Atherosclerotic aneurysms of the abdominal aorta are most frequently infra-renal, above the bifurcation of the aorta but may extend into common iliac arteries.
They may be of variable size but are often larger than 5-6 cm in diameter.
An atherosclerotic aneurysm is most frequently fusiform in shape and the lumen of aneurysm often contains mural thrombus.
Histologically, the wall of atherosclerotic aneurysm loses its normal arterial structure.
Instead, there is a predominance of fibrous tissue in the media and adventitia with mild chronic inflammatory reaction.
The intima and inner part of the media show remnants of atheromatous plaques and mural thrombus.
Effects: The clinical effects of atherosclerotic aneurysms are due to complications. These are as under:
1. Rupture: Rupture of the atherosclerotic aneurysm is the most serious and fatal complication.
The risk of rupture depends upon the size and duration of the aneurysm and the blood pressure.
Rupture of abdominal aneurysm may occur either into the peritoneum or into the retroperitoneum resulting in sudden and massive bleeding.
Occasionally, there may be a slow progressive leak from the aneurysm. A ruptured aneurysm is more likely to get infected.
2. Compression: The atherosclerotic aneurysm may press upon some adjacent structures such as compression of the ureter and erosion on the vertebral bodies.
3. Arterial occlusion: Atherosclerotic aneurysms of the abdominal aorta may occlude the inferior mesenteric artery, or there may be the development of occlusive thrombosis.
However, collateral circulation develops slowly and is nearly always sufficient so as not to produce the effects of ischaemia.
Thromboembolism is rather common in abdominal aneurysms.
Syphilitic (Luetic) Aneurysms:
Cardiovascular syphilis occurs in about 10% of cases of syphilis.
It causes arteritis—syphilitic aortitis and cerebral arteritis, both of which are already.
One of the major complications of syphilitic aortitis is a syphilitic or luetic aneurysm that develops in the tertiary stage of syphilis.
It usually manifests after the age of 50 years and is more common in men.
The predominant site of involvement is the thoracic aorta, especially in the ascending part and arch of the aorta.
It may extend proximally into the aortic valve causing aortic incompetence and may lead to syphilitic heart disease.
Less often, it may extend distally to involve the abdominal aorta.
Pathogenesis:
About 40% of cases of syphilitic aortitis develop syphilitic aneurysms.
The process begins from inflammatory infiltration around the vasa vasorum of the adventitia, followed by endarteritis obliterans.
This results in ischaemic injury to the media causing destruction of the smooth muscle and elastic tissue of the media and scarring.
Since syphilitic aortitis involves the proximal aorta maximally, aortic aneurysm is found most frequently in the ascending aorta and in the aortic arch.
Morphologic Features:
Syphilitic aneurysms occurring most often in the ascending part and the arch of the aorta are saccular in shape and usually 3-5 cm in diameter.
Less often, they are fusiform or cylindrical. The intimal surface is wrinkled and shows a tree-bark appearance.
When the aortic valve is involved, there is stretching and rolling of the valve leaflets producing valvular incompetence and left ventricular hypertrophy due to volume overload.
This results in a massively enlarged heart called ‘cor bovine.
Histologically, the features of healed syphilitic aortitis are seen.
The adventitia shows fibrous thickening with endarteritis obliterans of vasa vasorum.
The fibrous scar tissue may extend into the media and the intima. Rarely, spirochaetes may be demonstrable in syphilitic aneurysms.
Often, the mural thrombus is found in the aneurysm.
Effects:
The clinical manifestations are found much more frequently in syphilitic aneurysms than in atherosclerotic aneurysms. The effects include the following:
1. Rupture: Syphilitic aneurysm is likely to rupture causing massive and fatal haemorrhage into the pleural cavity, pericardial sac, trachea and oesophagus.
2. Compression: The aneurysm may press on the adjacent tissues and cause symptoms such as on the trachea causing dyspnoea, on the oesophagus causing dysphagia, on the recurrent laryngeal nerve leading to hoarseness; and erosion of vertebrae, sternum and ribs due to persistent pressure.
3. Cardiac dysfunction: When the aortic root and valve are involved, a syphilitic aneurysm produces aortic incompetence and cardiac failure. Narrowing of the coronary ostia may further aggravate the cardiac disease.
Dissecting Aneurysms And Cystic Medial Necrosis:
The term dissecting aneurysm is applied to a dissecting haematoma in which the blood enters the separated (dissected) wall of the vessel and spreads for varying distances longitudinally.
The most common site is the aorta which is an acute catastrophic aortic disease.
The condition occurs most commonly in men in the age range of 50 to 70 years.
In women, dissecting aneurysms may occur during pregnancy.
Pathogenesis:
The pathogenesis of dissecting an aneurysm is explained on the basis of weakened aortic media.
Various conditions causing weakening in the aortic wall resulting in dissection are as under:
Hypertensive state: About 90% of cases of dissecting aneurysms have hypertension which predisposes such patients to degeneration of the media in some questionable way.
Non-hypertensive cases: These are cases in whom there is some local or systemic connective tissue disorder e.g.
- Marfan’s syndrome is an autosomal dominant disease with a genetic defect in fibrillin which is a connective tissue protein required for elastic tissue formation.
- Development of cystic medial necrosis of Erdheim, especially in old age.
- Iatrogenic trauma during cardiac catheterisation or coronary bypass surgery.
- Pregnancy, for some unknown reasons.
Once medial necrosis has occurred, haemodynamic factors, chiefly hypertension, cause tears in the intima and initiate the dissecting aneurysms. The media is split at its weakest point by the inflowing of blood.
An alternative suggestion is that the medial haemorrhage from the vasa vasorum occurs first and the intimal tear follows it.
Further extension of an aneurysm occurs due to the entry of blood into the media through the intimal tear.
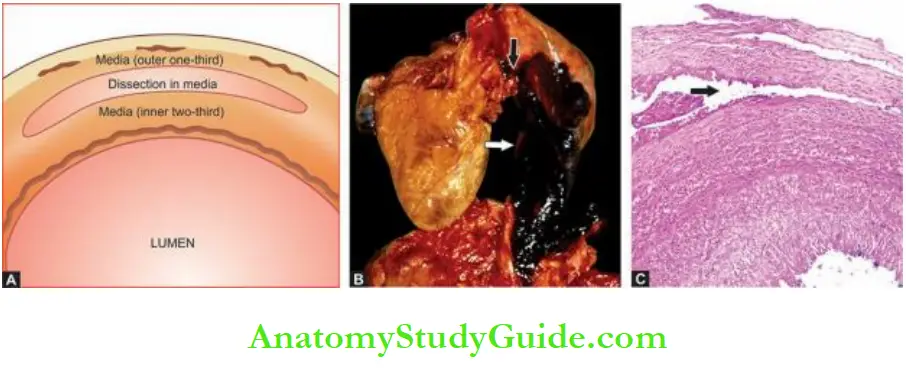
Morphologic Features:
Dissecting aneurysms differs from atherosclerotic and syphilitic aneurysms in having no significant dilatation.
Therefore, it is currently referred to as ‘dissecting haematoma’.
Dissecting an aneurysm classically begins in the arch of the aorta.
In 95% of cases, there is a sharply-incised, transverse or oblique intimal tear, 3-4 cm long, most often located in the ascending part of the aorta.
The dissection is seen most characteristically between the outer and middle third of the aortic media so that the column of blood in the dissection separates the intima and inner two-thirds of the media on one side from the outer one-third of the media and the adventitia on the other.
The dissection extends proximally into the aortic valve ring as well as distally into the abdominal aorta.
Occasionally, the dissection may extend into the branches of the aorta e.g. into the arteries of the neck, coronaries, renal, mesenteric and iliac arteries.
The dissection may affect the entire circumference of the aortic media or a segment of it.
In about 10% of dissecting aneurysms, a second intimal tear is seen in the distal part of the dissection so that the blood enters the false lumen through the proximal tear and re-enters the true lumen through the distal tear.
If the patient survives, the false lumen may develop endothelial lining and the ‘double-barrel aorta’ is formed.
Two classification schemes for dissections of thoracic aorta and intramural haematoma have been described:
DeBakey classification Depending upon the extent of aortic dissection
Type 1: Comprises 75% of cases; the intimal tear begins in the ascending aorta but dissection extends distally for some distance.
Type 2: Comprises 5% of cases and dissection is limited to the ascending aorta.
Type 3: Constitutes the remaining 20% of cases. In these cases, the intimal tear begins in the descending thoracic aorta near the origin of the subclavian artery and dissection extends distally.
2. Stanford classification Depending upon clinical management, these are divided into 2 types:
Type A (Proximal dissection): Involves the ascending aorta and includes type I and II of the above scheme because clinical management of DeBakey type I and II is not different.
Type B (Distal dissection): Limited to descending aorta and sparing the ascending aorta; it corresponds to DeBakey type III.
Histologically, the characteristic features of cystic medial necrosis are found.
These are as under:
- Focal separation of the fibromuscular and elastic tissue of the media.
- Numerous cystic spaces in the media contain basophilic ground substances.
- Fragmentation of the elastic tissue.
- Increased fibrosis of the media.
Effects
The classical clinical manifestation of a dissecting aneurysm is excruciating tearing pain in the chest moving downwards.
The complications arising from dissecting aneurysms are as under:
1. Rupture Haemorrhage from rupture of a dissecting aneurysm in the ascending aorta results in mortality in 90% of cases.
Most often, haemorrhage occurs in the pericardium; less frequently it may rupture into the thoracic cavity, abdominal cavity or retroperitoneum.
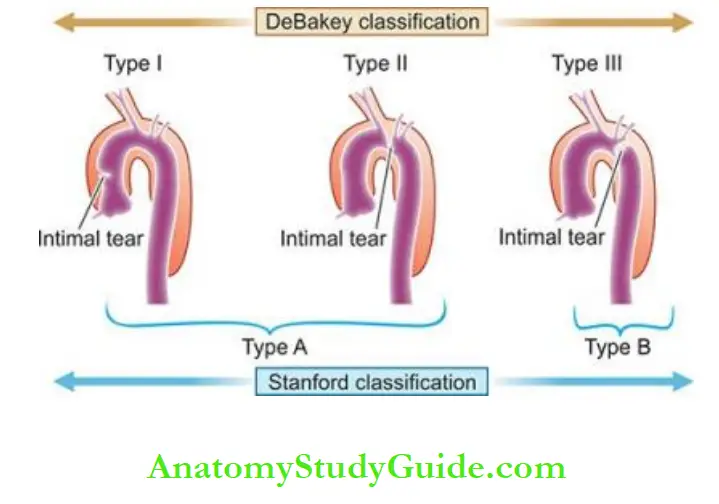
2. Cardiac disease: Involvement of the aortic valve results in aortic incompetence. Obstruction of coronaries results in ischaemia causing fatal myocardial infarction. Rarely, the dissecting aneurysm may extend into the cardiac chamber.
3. Ischaemia: Obstruction of the branches of the aorta by dissection results in ischaemia of the tissue supplied.
Thus, there may be renal infarction, cerebral ischaemia and infarction of the spinal cord.
Fibromuscular Dysplasia:
Fibromuscular dysplasia first described in 1976, is a non-atherosclerotic, non-inflammatory, hyperplastic disorder affecting medium-sized and small arteries, most often renal arteries.
Though the process may involve intima, media or adventitia, medial fibroplasia is more common.
Morphologic Features:
Grossly, the involvement is characteristically segmental affecting vessels in a bead-like pattern with intervening uninvolved areas.
Microscopically, the beaded areas show collections of smooth muscle cells and connective tissue. There is often rupture and retraction of the internal elastic lamina.
The main effects of renal fibromuscular dysplasia, depending upon the region of involvement, are renovascular hypertension and changes in renal atrophy.
Aneurysms:
An aneurysm is a permanent abnormal dilatation of a blood vessel due to congenital or acquired weakening or destruction of the vessel wall.
Based on pathogenetic mechanisms, aneurysms are atherosclerotic, syphilitic (luetic),
dissecting, mycotic and berry aneurysms; the last ones are seen in the circle of Willis in the base of the brain.
Atherosclerotic aneurysms are the most common and affect the abdominal aorta more often.
They may rupture or cause compression on adjacent tissues.
A syphilitic aneurysm occurs due to syphilitic aortitis and affects ascending and arch of the aorta.
Dissecting haematoma is often preceded by hypertension and affects the arch and ascending aorta most often.
Common Non-Neoplastic Diseases Of Veins
Here, two common non-neoplastic conditions of veins are discussed:
- Dilatations as varicosities (varicose veins)
- Inflammatory and thrombotic involvement as phlebothrombosis and thrombophlebitis and their clinical variants.
Varicosities:
Varicosities are abnormally dilated and tortuous veins. The veins of the lower extremities are involved most frequently, called varicose veins.
The veins of other parts of the body which are affected are discussed in related the lower oesophagus, the anal region (haemorrhoids,) and the spermatic cord (varicocele).
Varicose Veins:
Varicose veins are permanently dilated and tortuous superficial veins of the lower extremities,
especially the long saphenous vein and its tributaries.
About 10-12% of the general population develops varicose veins of the lower legs, with the peak incidence in the 4th and 5th decades of life.
Adult females are affected more commonly than the males, especially during pregnancy.
This is attributed to venous stasis in the lower legs because of compression on the iliac veins by the pregnant uterus.
Etiopathogenesis:
A number of etiologic and pathogenetic factors are involved in causing varicose veins.
These are as follows:
- Familial weakness of vein walls and valves is the most common cause.
- Increased intraluminal pressure due to prolonged upright posture e.g. in nurses, policemen, surgeons etc.
- Compression of iliac veins e.g. during pregnancy, intravascular thrombosis, growing tumour etc.
- Hormonal effects on smooth muscle.
- Obesity.
- Chronic constipation.
Morphologic Features:
The affected veins, especially of the lower extremities, are dilated, tortuous, elongated and nodular.
Intraluminal thrombosis and valvular deformities are often found.
Histologically, there is a variable fibromuscular thickening of the wall of the veins due to alternate dilatation and hypertrophy.
Degeneration of the medial elastic tissue may occur which may be followed by calcific foci.
Mural thrombosis is commonly present which may get organised and hyalinised leading to irregular intimal thickening.
Effects:
Varicose veins of the legs result in venous stasis which is followed by congestion, oedema, thrombosis, stasis, dermatitis, cellulitis and ulceration. Secondary infection results in chronic varicose ulcers.
Phlebothrombosis And Thrombophlebitis:
The terms ‘phlebothrombosis’ or thrombus formation in veins, and ‘thrombophlebitis’ or inflammatory changes within the vein wall occluded by venous thrombus, are used synonymously.
Etiopathogenesis:
Venous thrombosis that precedes thrombophlebitis is initiated by a triad of changes: endothelial damage, alteration in the composition of blood and venous stasis.
The factors that predispose to these changes are cardiac failure, malignancy, use of oestrogen-containing compounds, postoperative state and immobility due to various causes.
Morphologic Features:
The most common locations for phlebothrombosis and thrombophlebitis are the deep veins of the legs accounting for 90% of cases; it is commonly termed deep vein thrombosis (DVT).
Other locations are periprostatic venous plexus in males, pelvic veins in females, and near the foci of infection in the abdominal cavity such as acute appendicitis, peritonitis, acute salpingitis and pelvic abscesses.
Grossly, the affected veins may appear normal or may be distended and firm. Often, a mural or occlusive thrombus is present.
Histologically, the thrombus that is attached to the vein wall induces an inflammatory-reparative response beginning from the intima and infiltrating into the thrombi.
The response consists of mononuclear inflammatory cells and fibroblastic proliferation. In the late stage, the thrombus is either organised or resolved leading to a thick-walled fibrous vein.
Effects:
The clinical effects due to phlebothrombosis and thrombophlebitis may be local or systemic.
Local effects are oedema distal to occlusion, heat, swelling, tenderness, redness and pain.
Systemic effects are more severe and occur due to embolic phenomena, pulmonary thromboembolism being the most common and most important.
Other systemic manifestations include bacteraemia and septic embolisation to the brain, meninges, liver etc.
Clinical Variants Of Phlebothrombosis And Thrombophlebitis
A few clinical variants of phlebothrombosis are considered below:
1. Thrombophlebitis Migrans: Thrombophlebitis migrans or migratory thrombophlebitis or Trousseau’s syndrome is the term used for multiple venous thrombi that disappear from one site so as to appear at another site.
The condition is not a morphologic entity but a clinical one, seen most often in disseminated visceral cancers (e.g. cancer of the lungs, prostate, female reproductive tract, breast, pancreas and gastrointestinal tract) as part of paraneoplastic syndrome and is also found in nonbacterial thrombotic endocarditis.
2. Phlegmasia Alba Dolens: This term meaning ‘painful white leg’ refers to extensive swelling of the leg, occurring most frequently due to iliofemoral venous thrombosis.
It occurs most often in women during late pregnancy or following delivery when the pregnant uterus causes pressure on the iliofemoral veins or after extensive pelvic surgery.
Development of pulmonary embolism may occur due to the involvement of the inferior vena cava.
3. Phlegmasia Cerulea Dolens:
This term meaning ‘painful blue leg’ refers to markedly swollen bluish skin with superficial gangrene. It is a serious complication of massive iliofemoral venous thrombosis and decreased arterial blood flow.
4. Superior Vena Caval Syndrome:
Superior vena cava syndrome refers to obstruction of the superior vena cava. The obstruction results most often from external compression or from thrombosis.
Some of the common causes of superior vena cava syndrome are malignancy (especially lung cancer and lymphoma), syphilitic aortic aneurysm and tuberculous mediastinitis.
Clinical features include dilated veins of the neck and thorax, oedema of the face, neck and upper chest, visual disturbances and disturbed sensorium.
5. Inferior Vena Caval Syndrome: Inferior vena cava syndrome is the obstruction of the inferior vena cava.
Most often, obstruction results from thrombosis by extension from iliofemoral veins. Other causes of obstruction are external compression and neoplastic invasion.
Clinical features are oedema of the lower extremities, dilated leg veins and collateral venous channels in the lower abdomen.
Common Non-neoplastic Diseases of Veins:
Varicosities are abnormally dilated and tortuous veins.
Varicose veins are permanently dilated and tortuous superficial veins of the lower extremities, especially the long saphenous vein and its tributaries.
Phlebothrombosis is thrombus formation in veins, and thrombophlebitis is inflammatory changes within the vein wall.
Common Non-Neoplastic Diseases Of Lymphatics
Lymphatics are more commonly involved secondarily in the vicinity of an inflammatory or a neoplastic disease e.g.
lymphangitis and lymphoedema. A form of primary lymphoedema is also briefly discussed under lymphoedema below.
Tumours and tumour-like lesions of lymphatics were discussed along with those of blood vessels later.
Lymphangitis:
Inflammation of the lymphatics or lymphangitis may be acute or chronic.
Acute lymphangitis occurs in the course of many bacterial infections.
The most common organisms are β-haemolytic streptococci and staphylococci. Acute lymphangitis is often associated with lymphadenitis.
Grossly, the affected lymphatics are dilated and appear as cutaneous streaks.
Microscopically, the dilated lumen contains acute inflammatory exudate, cell debris and clotted lymph.
There is inflammatory infiltration into the perilymphatic tissues along with hyperaemia and oedema.
Acute lymphangitis generally heals completely.
Chronic lymphangitis occurs due to persistent and recurrent acute lymphangitis or from chronic infections like tuberculosis, syphilis and actinomycosis.
Histologically, there is permanent obstruction due to fibrosis of affected lymphatics called chronic lymphoedema.
Lymphoedema
Lymphoedema is the swelling of soft tissues due to a localised increase in the quantity of lymph.
It may be primary (idiopathic) or secondary (obstructive).
1. Primary (Idiopathic) Lymphoedema: Lymphoedema occurring without underlying secondary cause is called primary or idiopathic lymphoedema.
Its various types are as under:
1. Congenital lymphoedema: Congenital lymphoedema has further 2 subtypes—familial hereditary form (Milroy’s disease) and non-familial (simple) form.
- Milroy’s disease is a form of congenital and familial oedema generally affecting one limb but at times may be more extensive and involve the eyelids and lips. The disease is inherited as an autosomal dominant trait and is often associated with other congenital anomalies.
- The condition results from a developmental defect of lymphatic channels so that the affected tissue shows abnormally dilated lymphatics and the area shows a honey-combed appearance. Recurrent infection of the tissue causes cellulitis and fibrosis of lymphatic vessels.
- A simple congenital lymphoedema is a non-familial form with unknown aetiology. It is often associated with Turner’s syndrome and affects one member of the family. The pathologic changes are similar to those of Milroy’s disease.
2. Lymphoedema praecox: This is a rare form of lymphoedema affecting chiefly young females.
The oedema usually begins in the foot and progresses slowly upwards to involve the whole extremity. With the passage of time, the affected area becomes rough and the oedema is nonpitting.
The etiology is unknown but probably the condition is related to the female reproductive system because of preponderance in females and aggravation during menses.
2. Secondary (Obstructive) Lymphoedema:
This is a more common form of lymphoedema. Various causes of lymphatic obstruction causing lymphoedema are as under:
- Lymphatic invasion by a malignant tumour.
- Surgical removal of lymphatics e.g. in radical mastectomy.
- Post-irradiation fibrosis.
- Parasitic infestations e.g. in filariasis of lymphatics producing elephantiasis.
- Lymphangitis causes scarring and obstruction.
Obstructive lymphoedema: occurs only when the obstruction is widespread otherwise, collaterals develop.
The affected area consists of dilatation of lymphatics distal to obstruction with increased interstitial fluid.
With the passage of time, there is inflammatory scarring and the lymphatics become fibrosed with enlargement of the affected part.
Rupture of dilated large lymphatics may result in the escape of milky chyle into the peritoneum (hemoperitoneum), into the pleural cavity (chylothorax), into the pericardial cavity (hydropericardium) and into the urinary tract (chyluria).
Common Non-neoplastic Diseases of Lymphatics
- Inflammation of the lymphatics or lymphangitis may be acute or chronic.
- Lymphoedema is the swelling of soft tissues due to a localised increase in the quantity of lymph which may be primary (idiopathic) or secondary (obstructive)
Tumours And Tumour-Like Lesions
The majority of benign vascular tumours are malformations or hamartomas.
A hamartoma is a tumour-like lesion made up of tissues indigenous to the part but lacks the true growth potential of true neoplasms.
However, there is no clear-cut distinction between vascular hamartomas and true benign tumours and are often described together.
On the other hand, there are true vascular tumours which are of intermediate grade and there are frank malignant tumours.
Classification of vascular tumours and tumour-like conditions.
Benign Tumours
- Haemangioma
- Lymphangioma (cystic hygroma)
- Glomus tumour (glomangioma)
- Angiofibroma nose
Malformations And Reactive Proliferations
- Arteriovenous malformations
- Bacillary angiomatosis
Intermediate Grade Tumours
- Haemangioendothelioma
Malignant Tumours
- Angiosarcoma
- Kaposi’s sarcoma
1. Benign Tumours
Haemangioma:
Haemangiomas are quite common lesions, especially in infancy and childhood. The most common site is the skin of the face and mucosal surfaces.
Amongst the various clinical and histologic types, three important forms are described below.
Capillary Haemangioma:
These are the most common type.
Clinically, they appear as small or large, flat or slightly elevated, red to purple, soft and lobulated lesions, varying in size from a few millimetres to a few centimetres in diameter.
They may be present at birth or appear in early childhood. Strawberry birthmarks and ‘port-wine mark’ are some good examples.
The common sites are the skin, subcutaneous tissue and mucous membranes of the oral cavity and lips.
Less common sites are internal visceral organs like the liver, spleen and kidneys.
Histologically, capillary haemangiomas are well-defined but unencapsulated lobules.
These lobules are composed of capillary-sized, thin-walled, blood-filled vessels.
These vessels are lined by a single layer of plump endothelial cells surrounded by a layer of pericytes.
The vessels are separated by some connective tissue stroma.
Many capillary haemangiomas regress spontaneously within a few years.
Cavernous Haemangioma:
Cavernous haemangiomas are single or multiple, discrete or diffuse, red to blue, soft and spongy masses.
They are often 1 to 2 cm in diameter. They are most common in the skin (especially of the face and neck); other sites are mucosa of the oral cavity, stomach and small intestine, and internal visceral organs like the liver and spleen.
Histologically, cavernous haemangiomas are composed of thin-walled cavernous vascular spaces, filled partly or completely with blood.
The vascular spaces are lined by flattened endothelial cells. They are separated by scanty connective tissue stroma.
Cavernous haemangiomas rarely involute spontaneously.
Granuloma Pyogenicum:
Granuloma pyogenicum is also referred to as haemangioma of granulation tissue type.
True to its name, it appears as exophytic, red granulation tissue just like a nodule, commonly on the skin and mucosa of the gingiva or oral cavity.
A pregnancy tumour or granuloma gravidarum is a variant occurring on the gingiva during pregnancy and regresses
after delivery.
Granuloma pyogenic often develops following trauma and is usually 1 to 2 cm in diameter.
Histologically, it shows proliferating capillaries similar to capillary haemangioma but the capillaries are separated by abundant oedema and inflammatory infiltrate, thus resembling inflammatory granulation tissue.
Lymphangioma:
Lymphangiomas are lymphatic counterparts of vascular angiomas.
Lymphangiomas are congenital lesions which are classified as capillary, cavernous and cystic hygroma. Combinations are also often seen.
Capillary Lymphangioma
It is also called lymphangioma simplex. It is a small, circumscribed, slightly elevated lesion measuring 1 to 2 cm in diameter.
The common locations are the skin of the head and neck, axilla and mucous membranes. Rarely, these may be found in the internal organs.
Histologically, capillary lymphangioma is composed of a network of endothelium-lined, capillary-sized spaces containing lymph and often separated by lymphoid aggregates.
Cavernous Lymphangioma
It is more common than the capillary type. The common sites are in the region of the head and neck or axilla.
A large cystic variety called cystic hygroma occurs in the neck producing gross deformity in the neck.
Histologically, cavernous lymphangioma consists of large dilated lymphatic spaces lined by flattened endothelial cells and containing lymph.
Scanty intervening stromal connective tissue is present. These lesions, though benign, are often difficult to remove due to infiltration into adjacent tissues.
Glomus Tumour (Glomangioma)
Glomus tumour is an uncommon true benign tumour arising from contractile glomus cells that are present in the arteriovenous shunts (Sucquet-Hoyer anastomosis).
These tumours are found most often in the dermis of the fingers or toes under a nail; other sites are mucosa of the stomach and nasal cavity. These lesions are characterised by extreme pain.
They may be single or multiple, small, often less than 1 cm in diameter, flat or slightly elevated, red-blue, painful nodules.
Histologically, the tumours are composed of small blood vessels lined by endothelium and surrounded by aggregates, nests and masses of glomus cells.
The glomus cells are round to cuboidal cells with scanty cytoplasm.
The intervening connective tissue stroma contains some non-myelinated nerve fibres.
2. Malformations And Reactive Proliferations
Arteriovenous Malformations
An arteriovenous (AV) malformation is a communication between an artery and vein without an intervening capillary bed. It may be congenital or acquired type.
Congenital AV malformations have thick-walled vessels with hyalinisation and calcification.
Acquired AV malformations reveal changes mainly in the veins which are dilated and thick-walled.
Bacillary Angiomatosis And Peliosis Hepatis:
Bacillary angiomatosis is a tumour-like lesion due to reactive proliferation reported in association with HIV-AIDS with CD4+ T cell counts below 100/µl.
In fact, it is an opportunistic infection with gram-negative bacilli of the Bartonella genus.
The most common site of involvement is the skin and bones while a closely related condition peliosis hepatis is seen in the liver.
Grossly, the lesions on the skin are in the form of variable-sized red papules.
Histologically, lobules of proliferating blood vessels are seen lined by epithelioid endothelial cells having mild atypia.
Mixed inflammatory cell infiltrates with nuclear debris of neutrophils is present in these areas.
The condition is treated with antibiotics.
3. Intermediate Grade Tumours
Haemangioendothelioma
Haemangioendothelioma is a true tumour of endothelial cells, the behaviour of which is intermediate between a haemangioma and haemangiosarcoma.
It is found most often in the skin and subcutaneous tissue in relation to medium-sized and large veins.
Grossly, the tumour is usually a well-defined, grey-red, polypoid mass.
Microscopically, there is an active proliferation of endothelial cells forming several layers around the blood vessels so that vascular lumina are difficult to identify.
These cells may be epithelioid or spindled and may have variable mitotic activity. Reticulin stain delineates the pattern of cell proliferation inner to the basement membrane.
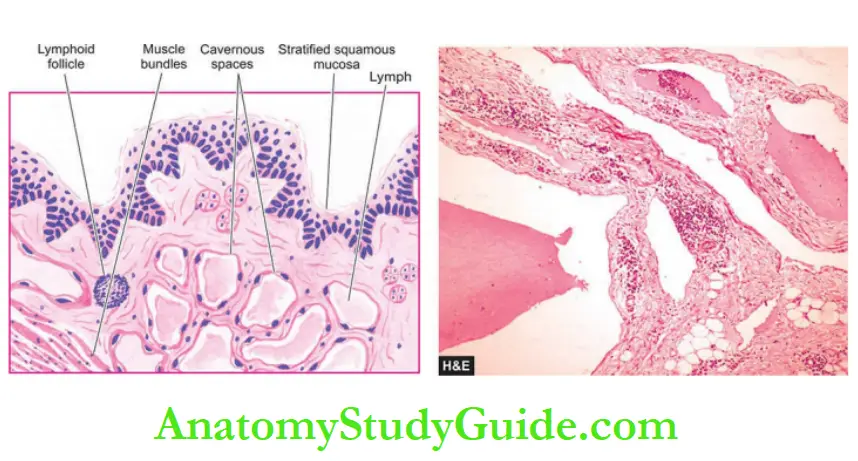
Haemangioblastoma is the term used for similar tumours occurring in the cerebellum. Kaposiform haemangioendothelioma is a rare aggressive vascular tumour of infancy, often accompanied by consumptive coagulopathy.
Microscopically, it shows nodules of spindled endothelial cells with low-grade anaplasia.
4. Malignant Tumours
Angiosarcoma
Also known as haemangiosarcoma and malignant haemangioendothelioma, it is a malignant vascular tumour occurring most frequently in the skin, subcutaneous tissue, liver, spleen, bone, lung and retroperitoneal tissues.
It can occur in both sexes and at any age. Hepatic
angiosarcomas are of special interest in view of their association with carcinogens like polyvinyl chloride, arsenical pesticides and radioactive contrast medium, thorotrast, used in the past.
Grossly, the tumours are usually bulky, pale grey-white, firm masses with poorly-defined margins. Areas of haemorrhage, necrosis and central softening are frequently present.
Microscopically, the tumours may be well-differentiated masses of proliferating endothelial cells around well-formed vascular channels, to poorly-differentiated lesions composed of plump, anaplastic and pleomorphic cells in solid clusters with poorly identifiable vascular channels.
These tumours invade locally and frequently have distant metastases in the lungs and other organs.
Lymphangiosarcoma is a histologically similar tumour occurring in obstructive lymphoedema of long duration.
Kaposi’s Sarcoma
Kaposi’s sarcoma is a malignant angiomatous tumour, first described by Kaposi, a Hungarian dermatologist, in 1872.
However, the tumour has attracted greater attention in the last two decades due to its frequent occurrence in patients with HIV/AIDS.
Classification:
Presently, four forms of Kaposi’s sarcoma are described:
1. Classic (European) Kaposi’s sarcoma: This is the form which was first described by Kaposi. It is more common in men over 60 years of age of Eastern European descent.
The disease is slow growing and appears as multiple, small, purple, dome-shaped nodules or plaques in the skin, especially on the legs. Involvement of visceral organs occurs in about 10% of cases after many years.
2. African (Endemic) Kaposi’s sarcoma: This form is common in equatorial Africa.
It is so common in Uganda that it comprises 9% of all malignant tumours in men.
It is found in younger ages, especially in boys and young men and has a more aggressive course than the classic form.
The disease begins in the skin but grows rapidly to involve other tissues, especially lymph nodes and the gut.
3. Epidemic (AIDS-associated) Kaposi’s sarcoma: This form is seen in about 30% of cases of AIDS, especially in young male homosexuals than the other high-risk groups.
The cutaneous lesions are not localised to the lower legs but are more extensively distributed involving mucous membranes, lymph nodes and internal organs early in the course of the disease.
4. Kaposi’s sarcoma in renal transplant cases: This form is associated with recipients of renal transplants who have been administered immunosuppressive therapy for a long time.
The lesions may be localised to the skin or may have widespread systemic involvement.
Pathogenesis:
Pathogenesis of Kaposi’s sarcoma is complex. It is an opportunistic neoplasm in immunosuppressed patients which has an excessive proliferation of spindle cells of vascular origin having features of both endothelium and smooth muscle cells
- Epidemiological studies have suggested a viral association implicating HIV and human herpesvirus 8 (HSV 8, also called Kaposi’s sarcoma-associated herpesvirus or KSHV).
- The occurrence of Kaposi’s sarcoma involves the interplay of HIV-1 infection, HHV-8 infection, activation of the immune system and secretion of cytokines (IL-6, TNF-α, GM-CSF, basic
fibroblast factor, and oncostatin M). - The higher incidence of Kaposi’s sarcoma in male homosexuals
is explained by the increased secretion of cytokines by their activated immune system. - Defective immunoregulation plays a role in its pathogenesis is further substantiated by observation of second malignancy (e.g. leukaemia, lymphoma and myeloma) in about one-third of patients with Kaposi’s sarcoma.
Morphologic Features Pathologically, all forms of Kaposi’s sarcoma are similar
Grossly, the lesions in the skin, gut and other organs form prominent, irregular, purple, domeshaped plaques or nodules.
Histologically, the changes are nonspecific in the early patch stage and more characteristic in the late nodular stage.
Early patch stage There are irregular vascular spaces separated by interstitial inflammatory cells and extravasated blood and haemosiderin.
Late nodular stage There are slit-like vascular spaces containing red blood cells and separated by spindle-shaped, plump tumour cells.
These spindle-shaped tumour cells are of endothelial origin.
Clinical Course:
The clinical course and biological behaviour of Kaposi’s sarcoma are quite variable.
The classic form of Kaposi’s sarcoma is largely confined to skin and the course is generally slow and insidious with long survival.
The endemic (African) and epidemic (AIDS-associated) Kaposi’s sarcoma, on the other hand, has a rapidly progressive course, often with widespread cutaneous as well as visceral involvement, and high mortality.
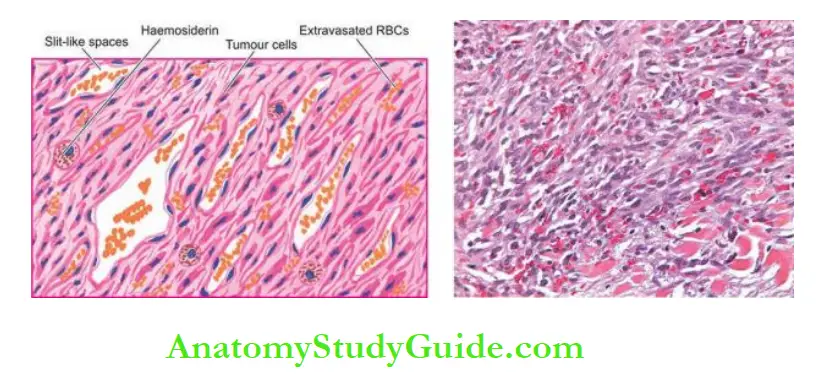
Tumours and Tumour-like Lesions
- The majority of benign vascular tumours are malformations or hamartomas. True vascular tumours are of intermediate grade and frank malignant tumours.
- Haemangiomas are quite common lesions on the skin and mucosal surfaces. These are 3 histologic types: capillary, cavernous and lymphangioma.
- Glomus tumour is an uncommon true benign tumour arising from contractile glomus cells and is common in the fingers.
- Haemangioendothelioma is a true tumour of endothelial cells, having an intermediate behaviour.
- Its common locations are skin and mucosal surfaces.
- Angiosarcoma is a malignant vascular tumour most frequent in the skin, subcutaneous tissue, liver, spleen, bone, lung and retroperitoneal tissues, and has widespread
metastases. - Kaposi’s sarcoma is an opportunistic neoplasm seen in immunosuppressed patients and has an association with HIV and HHV-8 infection
Leave a Reply