Hepatobiliary System
Functions Of Liver:
Question 1. Enumerate important functions of liver.
Answer:
Important functions of liver are listed:
Important functions of liver:
- Protein metabolism and urea formation
- Carbohydrate metabolism: Includes gluconeogenesis, glycogenolysis, and glycogenesis
- Lipid metabolism
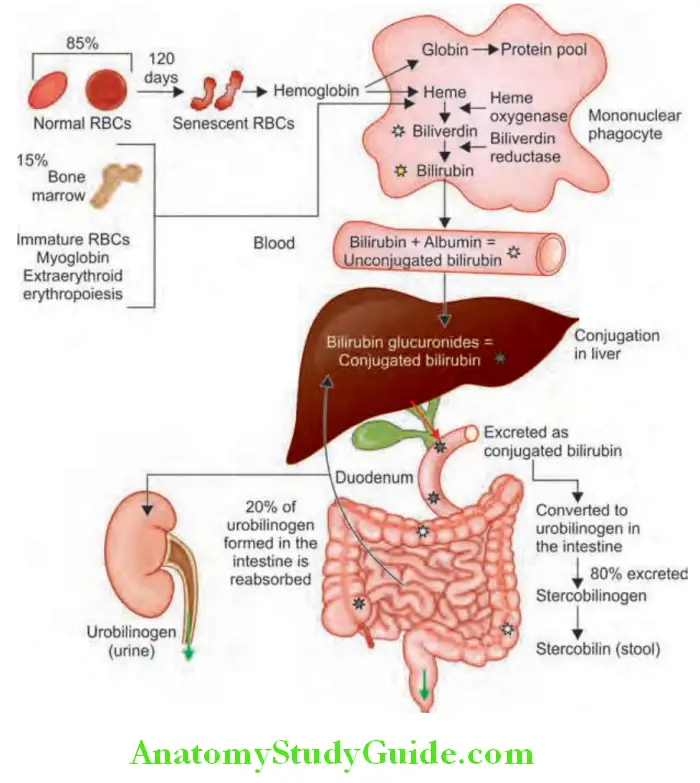
- Bilirubin formation from hemoglobin degradation
- Metabolism of vitamins and minerals
Read And Learn More: General Medicine Question And Answers
- Hormone metabolism
- Drug and alcohol metabolism
- Cholesterol metabolism
- Bile acid formation and bile secretion
- Synthesis of plasma proteins including coagulation factors
- Immunological function: Removal of gut endotoxins and foreign antigens
- Maintains core body temperature
- Maintains pH balance and correction of lactic acidosis
Liver Function Tests:
Question 2. Discuss the liver function tests. Discuss approach to jaundice.
Answer:
Liver Biochemistry:
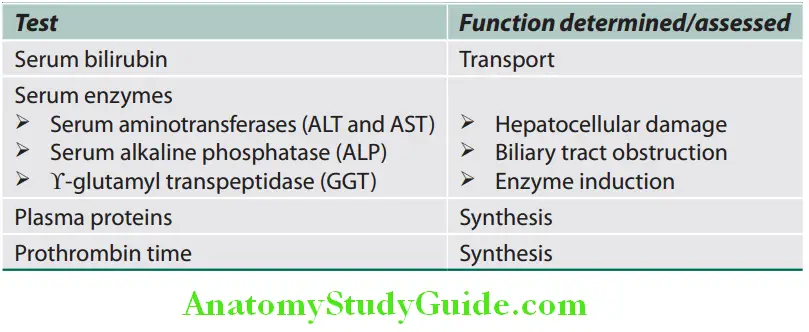
No single test alone can be used to assess liver function.
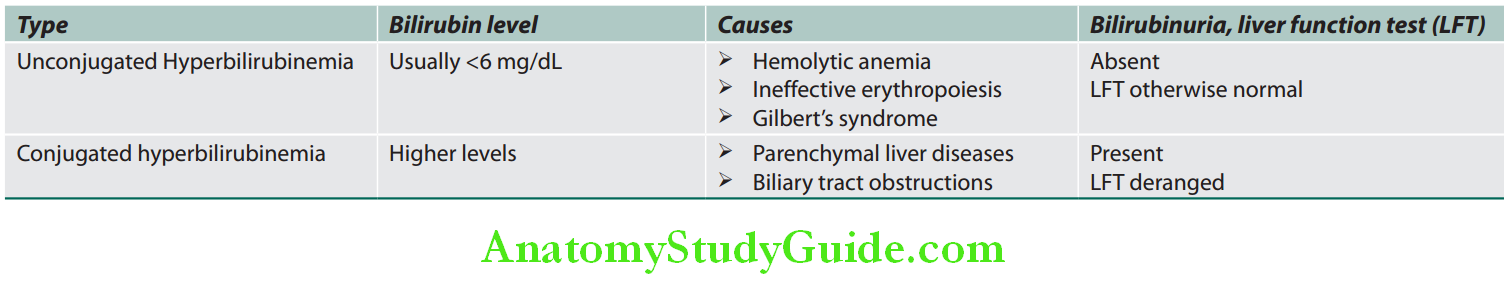
Serum Bilirubin:
- Normal values of total serum bilirubin are between 0.3 and 3 mg/dL, with 95% of a normal population falling between 0.2 and 0.9 mg/dL (almost all unconjugated). Bilirubin is a degradation product of hemoglobin and hem-containing proteins. Bilirubin metabolism is summarized.
- Total serum bilirubin = Conjugated (direct) + unconjugated (indirect) bilirubin.
- The presence of conjunctival icterus suggests a total serum bilirubin level of at least 0 mg/dL level below which is called anicteric jaundice, but does not allow differentiation between conjugated and unconjugated hyperbilirubinemia.
- Tea or cola-colored urine may indicate the presence of bilirubinuria and thus conjugated hyperbilirubinemia.
- Fluctuating hyperbilirubinemia is observed in gallstones, carcinoma of ampulla of Vater, chronic hepatitis, hemolytic anemias, and Gilbert’s syndrome.
Serum Enzymes:
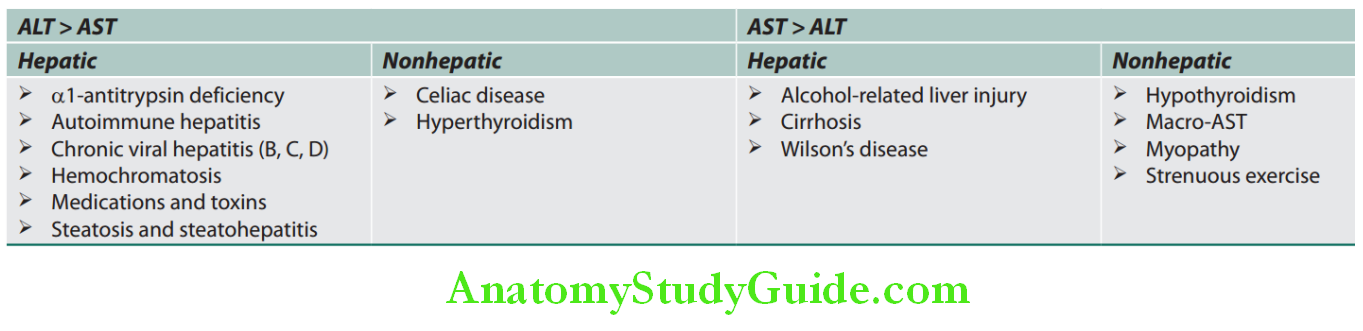
- Enzymes that reflect damage to hepatocytes— aminotransferases (transaminases)
- These enzymes are present in hepatocytes and leak into the blood with liver cell damage. These include two enzymes, namely: aspartate aminotransferase (AST/SGOT), and alanine aminotransferase (ALT/SGPT).
- Present in hepatocytes and leak into the blood with liver cell damage
- Normal value: 10–40 U/L.
- Poor correlation between the degree of liver cell damage and level of aminotransferases
- Aspartate aminotransferase: Mitochondrial and cytoplasmic isoenzymes. High concentration also in heart, muscle, kidney, and brain. Raised in hepatic necrosis, myocardial infarction, muscle injury, and congestive cardiac failure.
- Alanine aminotransferase: Cytosolic enzyme. More specific for liver injury.
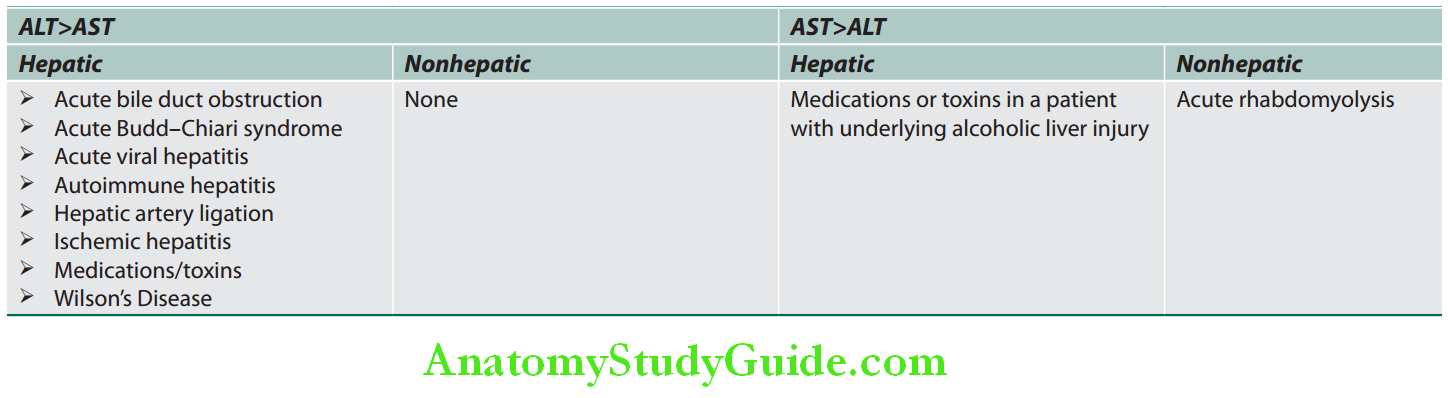
AST ALT ratio:
- AST: ALT >1: Chronic viral hepatitis and nonalcoholic fatty liver disease (NAFLD).
- AST: ALT ratio >2:1 is suggestive, while a ratio >3:1 is highly suggestive of alcoholic liver disease. A low level of ALT in the serum in alcoholic patients is due to an alcohol-induced deficiency of pyridoxal phosphate.
Causes of Elevated Serum Aminotransferases:
Chronic mild elevations (<150 U/L):
Acute severe elevations (>1,000 U/L):
Enzymes that reflect cholestasis:
These include three enzymes:
- Alkaline phosphatase (ALP)
- 5’-nucleotidase
- ϒ-glutamyl transpeptidase (GGT).
Question 3. Write short answer on causes/diseases with elevated/very high serum alkaline phosphatase.
Answer:
Alkaline phosphatase:
- Many distinct isoenzymes—liver, bone, kidney, placenta, small intestine, origin can be determined by electrophoretic separation.
- Normal serum level: 3–13 KA units (80–240 IU/L).
- Raised levels of liver-derived ALP are not totally specific for cholestasis.
- Low levels: Wilson’s disease, with fulminant hepatitis and hemolysis, possibly because of reduced activity of the enzyme owing to displacement of the cofactor zinc by copper. Ratio of ALP to total bilirubin of <2 is quite specific for Wilson’s disease.
- Raised serum ALP levels:
- <5 times: Hepatocellular jaundice
- >4 times:
- Obstructive jaundice (intrahepatic or extrahepatic obstruction)
- Infiltrative liver diseases, e.g., cancer, metastases, and amyloidosis
- Bone lesions with rapid bone turnover, e.g., Paget’s disease
- Primary biliary cirrhosis
ϒ-glutamyl transpeptidase (GGT):
- Microsomal enzyme is present in liver, renal tubules, pancreas, and intestine.
- Identify the source of isolated elevation in serum ALP (GGTP is normal in bone disease)
- Screening test for alcoholism: If ALP is normal, raised serum γ-GT is a good guide to alcohol intake of more than 60 g/day. (Detection of alcohol abuse in patients who deny it).
- Elevated GGT levels:
- Biliary obstruction
- Alcoholism
- Liver parenchymal damage
- Nonalcoholic fatty liver
- Other causes: Chronic obstructive lung disease, diabetes mellitus, hyperthyroidism, obesity, and renal failure.
- Patients taking phenytoin, barbiturates, and antiretroviral therapy—nonnucleoside reverse transcriptase inhibitors and abacavir
5-nucleotidase:
- Microsomal enzyme has similar significance as that of GGT.
- 5′NT levels are not increased in bone disease but are increased in hepatobiliary disease.
Lactate dehydrogenase (LDH): Not useful in diagnosis of liver diseases
- Moderate elevations: Ischemic hepatitis and hepatic metastasis.
- ALT/LDH ratio >5 suggests ischemic hepatitis while ratio <5 is seen with paracetamol toxicity.
Biosynthetic Function of the Liver:
Tests that measure synthetic function of the liver.
Plasma Proteins:
Serum albumin:
- It is synthesized exclusively in liver and used as a marker of synthetic function.
- Normal serum albumin level: 4–5.5 g/100 mL and albumin has a half-life of around 20 days.
Serum albumin Significance:
- Low levels: Serum albumin is an excellent marker of hepatic synthetic function. Hypoalbuminemia is observed in chronic liver diseases such as cirrhosis and chronic hepatitis and usually reflects severe liver damage and decreased albumin synthesis. A falling serum albumin level in liver disease is a bad prognostic sign.
- Apart from chronic liver disease, it may be observed in chronic inflammation, sepsis, expanded plasma volume, and renal or gastrointestinal (GI) loss.
Serum globulins:
- They constitute a group of proteins made up ofϒ-globulins (immunoglobulins) synthesized by B lymphocytes and α- and β-globulins synthesized primarily by hepatocytes.
- Normal serum globulin level: 5–5 g/100 mL.
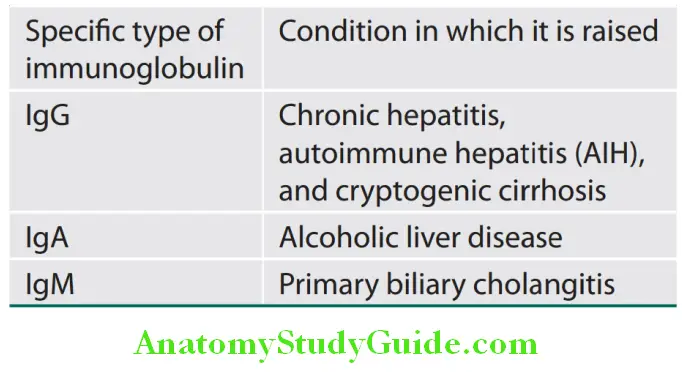
- Significance: ϒ-globulins are increased in chronic liver disease (e.g., chronic hepatitis and cirrhosis). Increase in the concentration of specific isotypes of ϒ-globulins (immunoglobulins) is helpful in the recognition of certain chronic liver diseases.
- Cirrhosis: Plasma proteins show decrease in albumin and increase in ϒ-globulin.
Specific types of elevated immunoglobulin and its associated conditions:
Coagulation factors:
- Liver produces all the coagulation factors, except factor VIII.
- Vitamin K is required for the activation of coagulation factors II, VII, IX, and X.
- The coagulation factors have short half-life time. Thus, measurement of the clotting factors is the single best measure of hepatic synthetic function and useful in both the diagnosis and assessing the prognosis of acute parenchymal liver disease.
- Prothrombin time:
- Prothrombin time depends on factors 1, 2, 4, 7, and 10.
- Normal value is 11–15 seconds.
- Prothrombin time collectively measures factors 2, 5, 7, and 10.
- Causes of prolonged prothrombin time are listed.
- Unlike the serum albumin, the prothrombin time allows an assessment of current hepatic synthetic function; factor VII has the shortest serum half-life (6 hours) of all the clotting factors.
Read And Learn More: General Medicine Question And Answers
Causes of prolonged prothrombin time:
- Severe liver damage: Acute hepatitis (e.g., viral hepatitis), cirrhosis
- Deficiency of vitamin K
- Obstructive jaundice that reduces vitamin K absorption
- Fat malabsorption
- Poor intake
- Antibiotic therapy which produces destruction of vitamin
- Producing commensals
- Disseminated intravascular coagulation
- Drugs and toxins: Warfarin, rivaroxaban, apixaban, edoxaban, dabigatran, viper envenomation, anticoagulant rodenticide poisoning
Ceruloplasmin:
- It is an acute-phase reactant synthesized by the liver.
- In blood, it binds to copper and acts as a major carrier for copper.
- Normal plasma level: 20–60 mg/dL
- Causes of elevated levels: Infections, liver diseases, obstructive jaundice, rheumatoid arthritis, and pregnancy.
- Causes of decreased levels: Wilson’s disease (due to decreased rate of synthesis), neonates, Menkes disease, kwashiorkor, marasmus, protein-losing enteropathy, and copper deficiency.
Cholesterol:
- It is synthesized in the liver. Advanced liver disease may be associated with very low cholesterol. However, primary biliary cholangitis (PBC) may be associated with markedly raised cholesterol. Similarly, a low urea level also indicates severe liver dysfunction. Liver functions tests and their significance are summarized.
Serum autoantibodies:
- Antimitochondrial antibody (AMA): Antinuclear, smooth muscle (actin), liver/kidney microsomal antibodies, antinuclear cytoplasmic antibodies (ANCA).
Summary of main liver function tests and its signifiance:
Urine Tests:
Bilirubin in urine:
- Normally, bilirubin cannot be detected in urine.
- In unconjugated hyperbilirubinemia, urine does not contain bilirubin (acholuric jaundice). Thus, absence of bilirubin in urine in a jaundiced patient suggests unconjugated hyperbilirubinemia.
- In conjugated hyperbilirubinemia, urine contains bilirubin.
- Thus, bilirubinuria in a jaundiced patient points to conjugated hyperbilirubinemia (hepatobiliary disease).
- Urinary bilirubin is detected by Fouchet’s test.
Urine urobilinogen:
- Urobilinogen is normally present in urine in trace amounts (1–2 mg/dL) and is insufficient to cause a significant positive reaction.
- Causes of increased urobilinogen in urine:
- Causes of absent urobilinogen: In obstructive jaundice (bilirubinuria present), bilirubin does not reach the intestine and so is not converted into urobilinogen.
- Urinary urobilinogen is detected by Ehrlich’s aldehyde test.
Causes of increased urobilinogen in urine:
Hemolytic anemias (without bilirubin in urine):
- Thalassemia
- Sickle-cell anemia
- Hereditary spherocytosis
Liver diseases (bilirubinuria present):
- Preicteric phase of infective hepatitis
- Drugs or toxic hepatitis
- Cirrhosis
α-Fetoprotein (AFP):
- α-fetoprotein is normally produced by fetal liver cells and its levels falls to low levels after birth.
- Causes of elevated levels ofα-fetoprotein are:
- Hepatocellular carcinoma (HCC)
- Carcinomas of stomach, pancreas, gallbladder, bile ducts, and lungs
- Teratomas
Diagnostic Procedures:
Endoscopic Retrograde Cholangiopancreatography:
Question 4. Write short note on endoscopic retrograde cholangiopancreatography.
Answer:
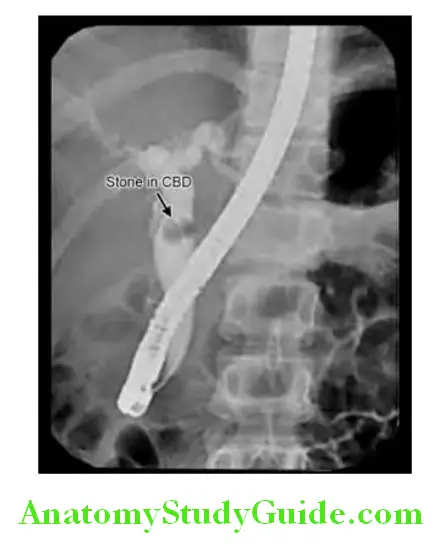
Endoscopic retrograde cholangiopancreatography (ERCP) is a technique used to outline the biliary and pancreatic ducts.
Diagnostic Procedure:
An endoscope is passed into the second part of the duodenum and cannulation of the ampulla. Contrast is injected into the biliary tree and the patient is screened radiologically. ERCP with stone in the common bile duct (CBD).
Diagnostic Uses of ERCP:
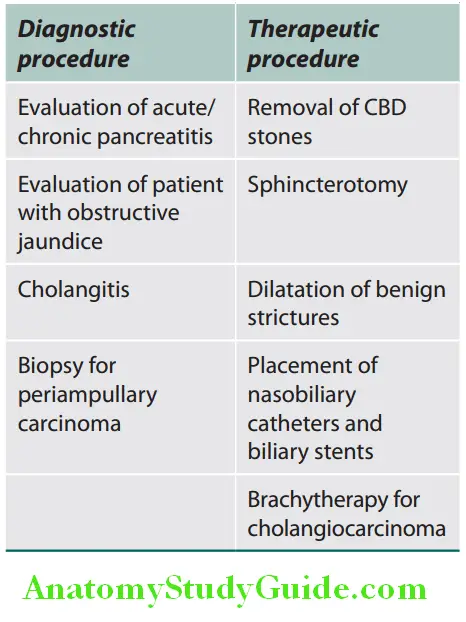
Diagnostic Complications:
The complication rate in diagnostic ERCP is 2–3%. Complications include pancreatitis, cholangitis, bleeding, and duodenal perforation.
Magnetic Resonance Cholangiopancreatography:
Question 5. Write short note on magnetic resonance cholangiopancreatography.
Answer:
Magnetic resonance cholangiopancreatography (MRCP) is a noninvasive technique largely replacing diagnostic (but not therapeutic) ERCP.
Technique:
A heavily T2-weighted sequence enhances visualization of the water-filled biliary (intrahepatic ducts) and pancreatic ducts (extrahepatic ducts). It produces high-quality images of ductal anatomy.
Advantages of MRCP over ERCP Indications:
- Diagnosis of bile duct obstruction and pancreatic duct abnormalities, e.g., choledocholithiasis, malignant obstruction of bile and pancreatic ducts, congenital anomalies, and chronic pancreatitis.
- Unsuccessful ERCP or a contraindication to ERCP (as in patients with cardiorespiratory compromise, renal failure).
Advantages of MRCP over ERCP:
- No need for contrast media or ionizing radiation
- Images can be acquired faster
- Less operator dependent
- No risk of pancreatitis
Endoscopic Ultrasound:
Question 6. Write a short note on endoscopic ultrasound.
Answer:
Endoscopic Ultrasound Procedure:
In endoscopic ultrasound (EUS), a small high-frequency ultrasound probe is placed on the tip of an endoscope and placed by direct vision into the duodenum.
Endoscopic Ultrasound Advantages:
Gradually replacing diagnostic ERCP:
- Close proximity of the ultrasound probe to the pancreas and biliary tree allows high-resolution ultrasound imaging.
- Accurate staging of small, potentially operable, pancreatic tumors (e.g., neuroendocrine tumors) can be done.
- It is less invasive method for bile duct imaging.
Endoscopic Ultrasound Uses:
Diagnostic:
- Imaging pancreatic and biliary diseases, e.g., choledocholithiasis, pancreatic and biliary cancers, and cystic lesions of the pancreas
- Ampullary carcinoma: To know the local extension of tumor and regional lymph node metastasis that cannot be evaluated
by ERCP - Performing fine-needle aspiration under EUS guidance from suspicious lesions for confirmation of malignancy
Therapeutic:
- Increasingly used for guided interventions:
- To reduce pain in patients with unresectable pancreatic carcinoma, for injecting bupivacaine and alcohol into the celiac ganglia.
- Endoscopic management of pancreatic pseudocysts.
Endoscopic Ultrasound Disadvantages:
- Cost is high.
- High degree of training is required.
Liver Biopsy:
Question 7. Write short note on the indications, significance, and complications of liver biopsy.
Answer:
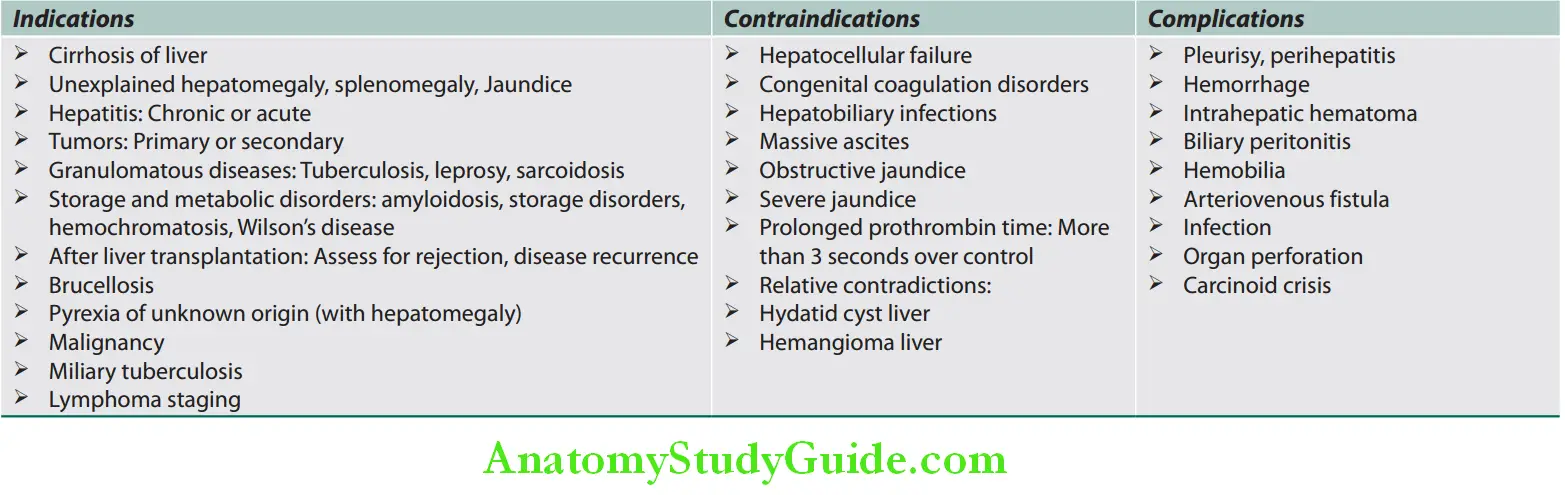
Percutaneous liver biopsy is an invasive procedure that can be performed with or without radiographic guidance. Indications, contraindications, and complications of liver biopsy.
Indications, contraindications, and complications of liver biopsy:
Jaundice:
Question 8. List the causes of jaundice. How will you arrive at the etiology of jaundice? Give the points of differentiation in clinical features and investigations.
Answer:
Jaundice Defiition:
Jaundice (icterus) is defied as yellowish pigmentation of skin, mucus membranes, and sclera due to increased levels of bilirubin in the blood. Th scleral involvement is because of its rich elastic tissue that has special affity for bilirubin.
- Normal serum bilirubin level: In normal adults, it ranges from 0.3 to 2 mg/dL.
- Jaundice is clinically detected when the serum bilirubin level is above 0–5 mg/dL. With severe disease, the levels may be as high as 30–40 mg/dL.
- Latent jaundice is the term used when serum bilirubin is more than 2 mg/dL but less than 5mg/dL.
- Carotenemia is characterized by yellowish pigmentation of skin by carotene but not of sclera. Quinacrine consumption also causes yellowish discoloration of skin and mucus membranes.
Classifiation of Jaundice:
Question 9. Write short note on causes of indirect/unconjugated hyperbilirubinemia.
Answer:
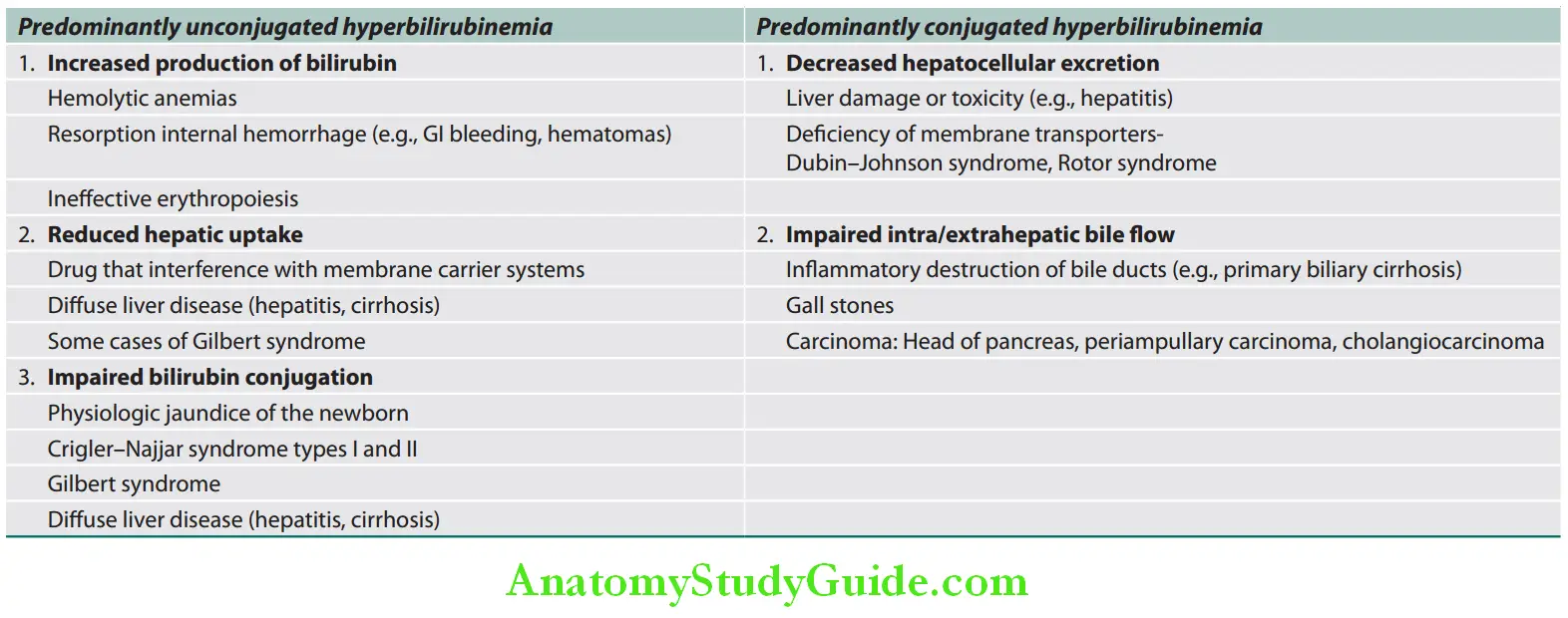
Jaundice can be classified in two ways:
1. Based on the underlying cause:
- Predominantly unconjugated hyperbilirubinemia
- Predominantly conjugated hyperbilirubinemia.
Classification of jaundice:
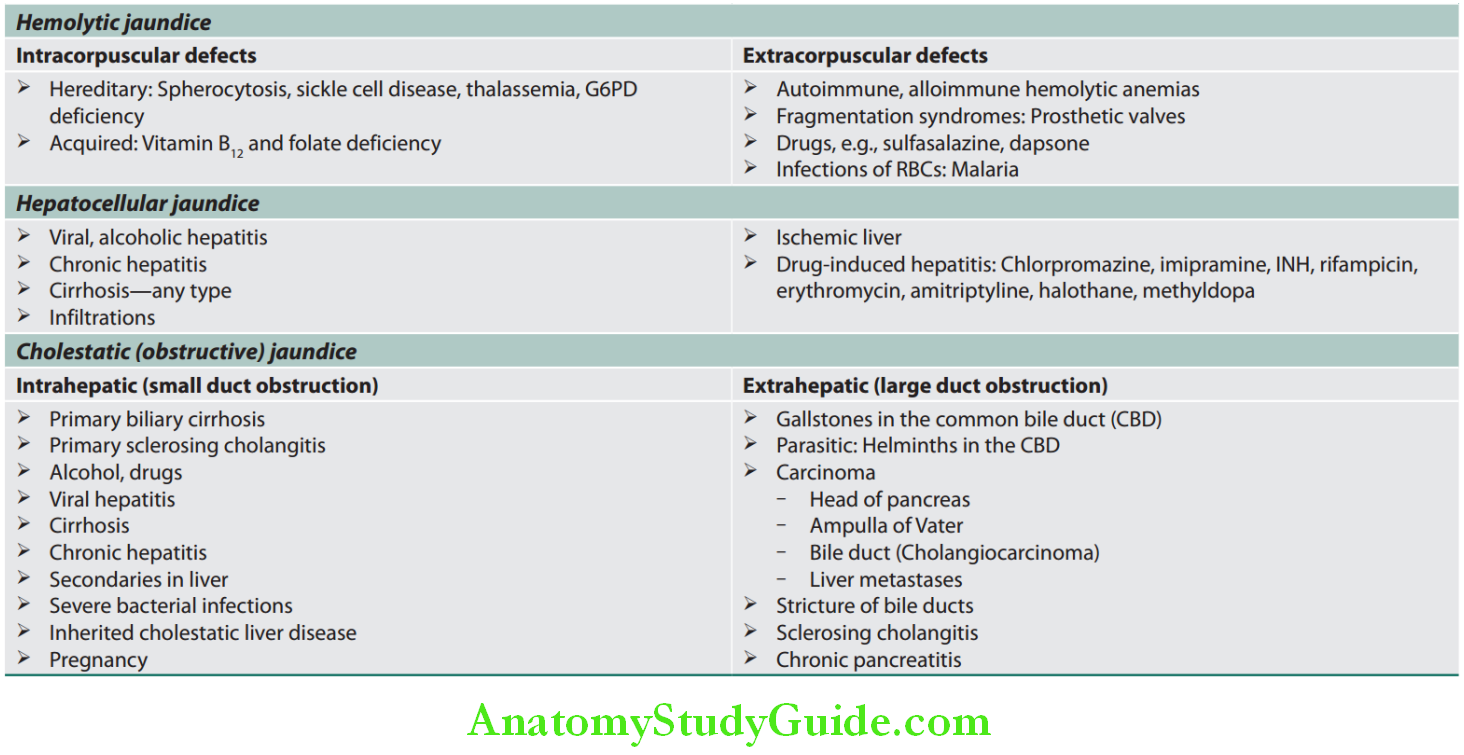
2. Based on pathological mechanism:
- Hemolytic (prehepatic) jaundice
- Hepatocellular jaundice (hepatic)
- Obstructive jaundice (posthepatic).
Classification of jaundice based on the pathological mechanism:
Hemolytic Jaundice:
Question 10. Write short note on prehepatic jaundice.
Answer:
- Increased destruction of red blood cells or their precursors causes increased production of bilirubin.
- Unconjugated bilirubin accumulates in the plasma and results in jaundice.
- Jaundice is usually mild because normal liver can easily handle the increased bilirubin production.
Question 11. How do you clinically differentiate hemolytic jaundice, hepatocellular jaundice, and obstructive (cholestatic) jaundice?
Answer:
Hemolytic jaundice Clinical features:
- Pallor due to anemia
- Mild jaundice without any signs of liver disease
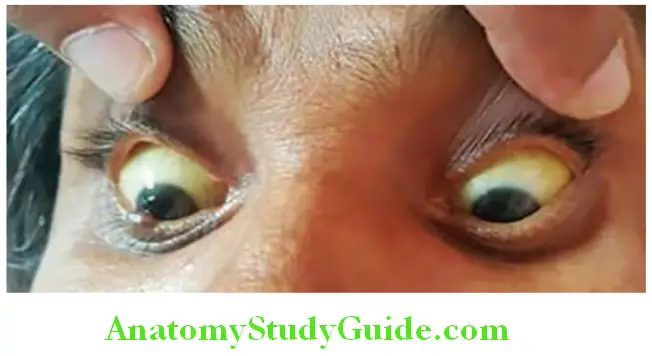
- Hepatosplenomegaly due to increased activity of reticuloendothelial system.
- Gallstones and leg ulcers may be seen depending on the cause of anemia.
- Dark stools due to increased stercobilinogen in stool
- Urine turns dark yellow on standing. This is due to increased urobilinogen being converted to urobilin in urine.
Hemolytic jaundice Investigations:
- Peripheral smear—shows features of hemolysis
- Predominantly unconjugated hyperbilirubinemia. Serum bilirubin is raised (<6 mg%).
- No bilirubin in urine, because the unconjugated bilirubin is not water soluble and cannot pass into the urine; hence the term “acholuric jaundice”
- Urinary urobilinogen is increased (more than 4 mg/24 hours).
- Other LFTs (e.g., serum ALP, transferases, and albumin) are normal; however, LDH is increased.
Hepatocellular Jaundice:
- Hepatocellular jaundice occurs as a consequence of parenchymal liver disease which leads to an inability of the liver to transport bilirubin across the hepatocyte into the bile.
- Defect in bilirubin transport across the hepatocyte may occur at any point between the uptake of unconjugated bilirubin into the hepatocyte and transport of conjugated bilirubin into biliary canaliculi.
- In hepatocellular jaundice, both unconjugated and conjugated bilirubin level rise in the blood
Hemolytic jaundice Investigations:
- Raised transaminases (AST and ALT): Acute jaundice with AST > 1000 U/L is highly suggestive of an infectious cause (e.g. hepatitis A, B), drugs (e.g., paracetamol) or hepatic ischemia.
- Imaging
- Liver biopsy
Cholestatic (Obstructive/Surgical) Jaundice:
Question 12. Write short note on causes and features of obstructive jaundice.
Answer:
- Cholestasis means failure of bile flow. Its cause may be anywhere between hepatocyte and duodenum.
- Cholestatic jaundice is usually a “surgical jaundice” meaning a cause that requires surgical intervention.
- Cholestasis can be intrahepatic or extrahepatic.
- Consequences of cholestasis:
- Retention of bile acids and bilirubin in the liver and blood
- Deficiency of bile acids in the intestine

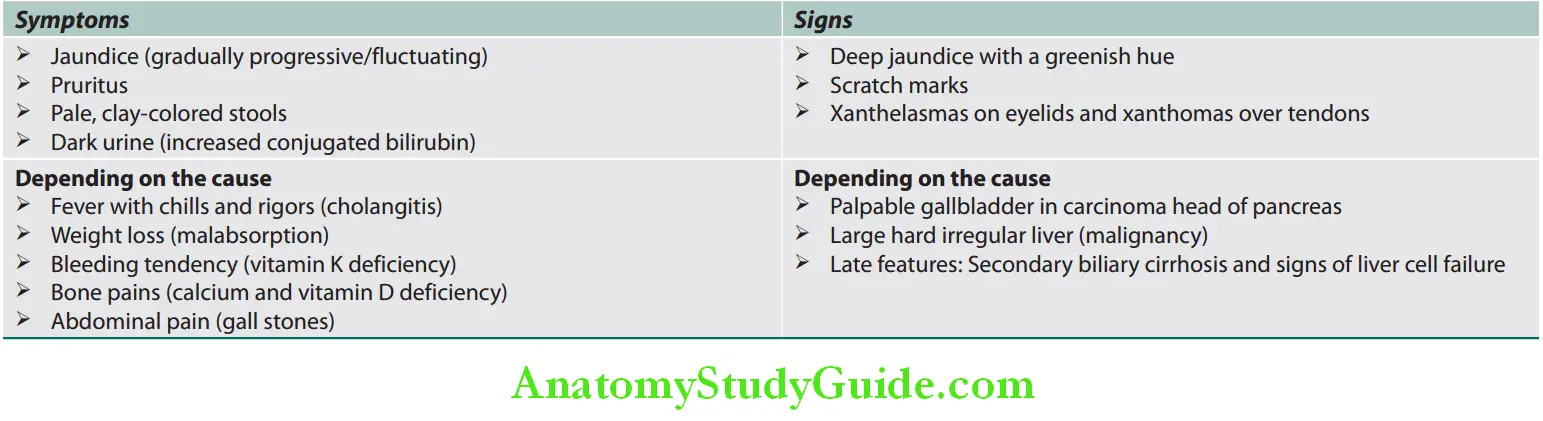
Cholestatic Clinical features:
Symptoms and signs of cholestatic jaundice:
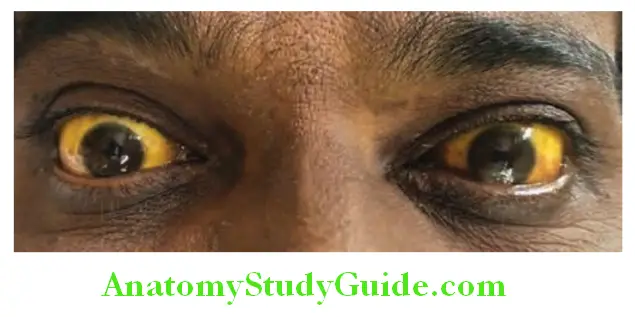
Cholestatic Investigations:
Serum findings:
- Serum bilirubin is markedly raised and is predominantly conjugated hyperbilirubinemia
- Serum ALP is markedly raised (3–4 times that of normal).
- Minimal biochemical changes of liver parenchymal damage.
- Antimitochondrial antibody (in primary biliary cholangitis).
Urine findings:
- Bilirubin present
- Urobilinogen absent
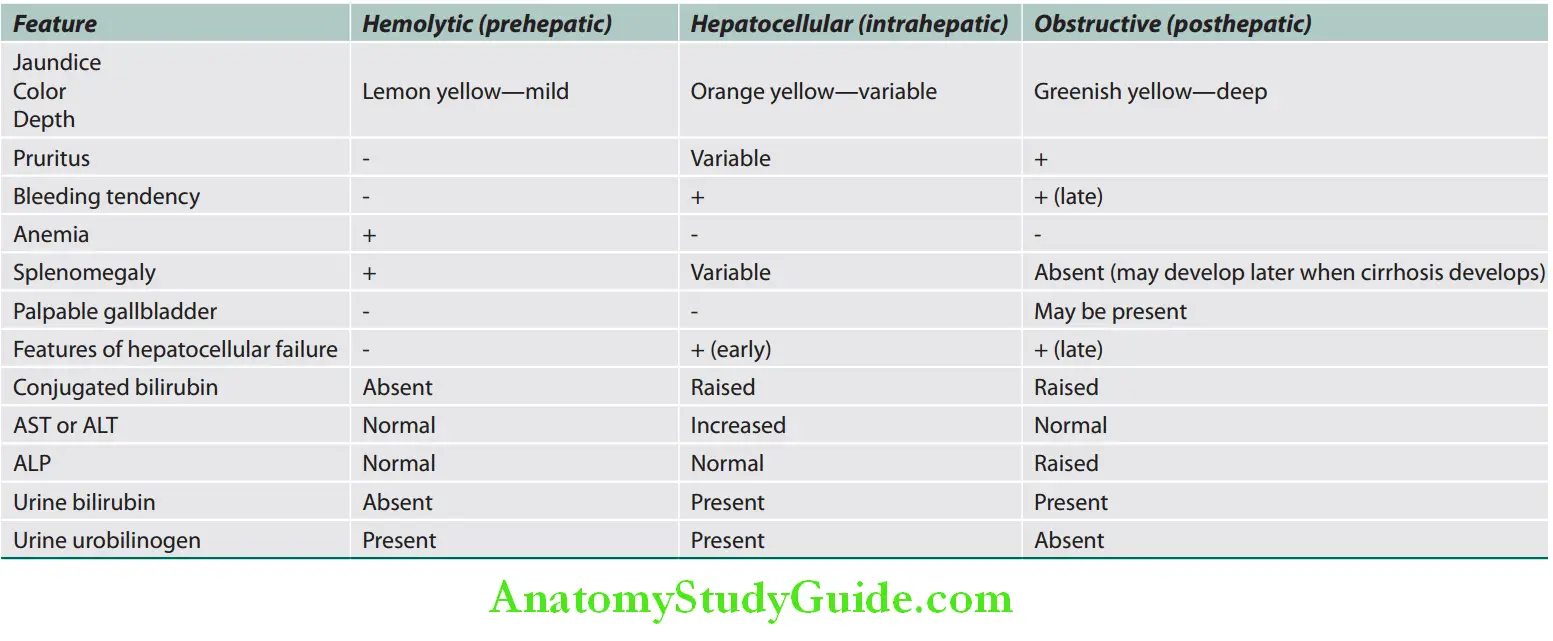
Clinical features useful in differentiating different types of jaundice are listed in Table:
Clinical features useful in diffrentiating diffrent types of jaundice:
Congenital Nonhemolytic Hyperbilirubinemias:
Question 13. List congenital nonhemolytic hyperbilirubinemias.
Answer:
Various congenital nonhemolytic hyperbilirubinemias are listed in Table:
Congenital nonhemolytic hyperbilirubinemias:
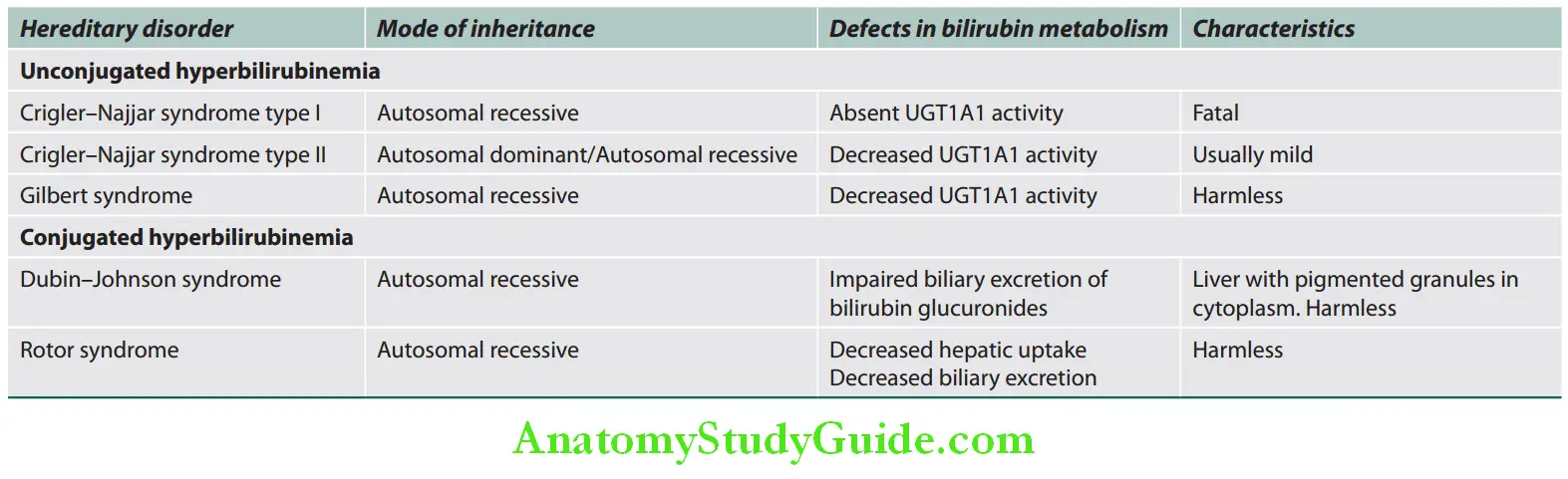
- Individuals with Crigler–Najjar or Gilbert syndrome cannot ConjuGate bilirubin.
- Individuals with Rotor syndrome or Dubin–Johnson syndrome cannot get RiD of DiRect bilirubin.
Gilbert Syndrome:
- Relatively common, autosomal recessive, harmless, inherited disorder
- Etiology: Mutations in UGT1 gene—inadequate synthesis of UGT1A1 enzyme (about 30% of normal)
- Clinical features:
- More common in males
- Usually asymptomatic, jaundice is incidentally detected.
- Mild, chronic unconjugated fluctuating hyperbilirubinemia.
- No other functional derangements
- Severity of jaundice increases with infections, fatigue, exertion, and fasting
- Physical examination is otherwise normal.
- Investigations:
- Unconjugated hyperbilirubinemia (less than 6 mg %)—raised bilirubin levels during fasting is the most common diagnostic tool.
- Urine: Increased urobilinogen and absent bilirubinuria
- Peripheral smear, reticulocyte count, and serum haptoglobin: Normal
Gilbert Syndrome Treatment: Usually no treatment is required. Glucuronosyltransferase activity may be increased by administering phenobarbital 60 mg BD.
Crigler–Najjar Syndrome Type 1:
- Rare, autosomal recessive disorder, invariably fatal
- Etiology: Due to complete absence of hepatic UGT1A
- Chronic, severe, unconjugated hyperbilirubinemia with severe jaundice, icterus and death secondary to kernicterus within 18 months of birth.
- Bile does contain conjugated bilirubin; hence it is colorless.
- Liver is morphologically normal by light and electron microscopy.
Crigler–Najjar Syndrome Type 1 Treatment: Daily phototherapy and liver transplantation. Phenobarbital has no effect.
Crigler–Najjar Syndrome Type 2:
- Less severe, nonfatal disorder, also known as Arias syndrome
- Autosomal recessive inheritance in most cases
- Partial deficiency of UGT1A1 enzyme (10% of normal).
- Jaundice is milder than type I and does not develop kernicterus
Crigler–Najjar Syndrome Type 2 Treatment: Treatment includes ultraviolet light therapy and liver transplantation. Phenobarbital treatment can improve bilirubin glucuronidation by inducing hypertrophy of hepatocellular endoplasmic reticulum.
Dubin–Johnson Syndrome:
- Benign autosomal recessive disorder
- Etiology: Complete absence of the multidrug resistance protein 2 (MRP2) which is required for secretion of conjugated bilirubin from hepatocytes into canaliculThis leads to defect in hepatocellular excretion of bilirubin glucuronides across biliary canalicular membrane.
- Clinical features: Chronic, recurrent conjugated hyperbilirubinemia, generally after puberty.
- Investigations:
- Conjugated hyperbilirubinemia (usually 2–5 mg/dL)
- Bromosulfthalein (BSP) clearance—impaired with reflux into blood at 90 minutes
- Bilirubinuria
- Gallbladder is usually not visualized on oral cholecystography.
- Liver biopsy: Dark pigment in centrilobular hepatocytes, coarse melanin-like pigmented granules within the enlarged lysosomes, present in the cytoplasm. Pigment composed of polymers of epinephrine metabolites.
No treatment is required in most cases. Most of the patients have a normal life expectancy.
Rotor Syndrome:
- Rare, autosomal recessive, asymptomatic conjugated hyperbilirubinemia.
- Defective organic anion transport proteins; (OATP) 1B1 and 1B3 in hepatocytes → impaired transport and reduced storage capacity of conjugated bilirubin
- Clinical presentation—mild jaundice.
- Investigations:
- Conjugated hyperbilirubinemia
- Bilirubinuria
- BSP clearance test—impaired without reflux back into blood
- Gallbladder is visualized on oral cholecystography.
- Liver is morphologically normal. No pigmentation
Charcot’s Triad:
Question 14. Write short note on Charcot’s triad.
Answer:
It consists of following in the presence of stones in bile ducts:
- Pain in the right hypochondrium
- Intermittent or persistent jaundice
- Fever with chills and rigors due to acute cholangitis.
Reynolds’ pentad adds mental status changes and sepsis to the triad.
Courvoisier’s Law:
Question 15. Describe Courvoisier’s law.
Answer:
In obstruction of CBD due to a stone, the gallbladder as a rule is impalpable (no distension). This is because the gallbladder is usually already shriveled, fibrotic, and nondistensible and hence will not be palpable.
In obstruction from other causes (e.g., carcinoma head of pancreas) distension of the gallbladder is common and hence gallbladder may be palpable.
Exceptions of Courvoisier’s law:
- Double impaction: Stones, simultaneously occluding the cystic duct and the distal.
- Pancreatic calculus obstructing the ampulla of Vater
- Oriental cholangiohepatitis
- Periampullary carcinoma in patients with cholecystectomy
- Mirizzi syndrome
Viral Hepatitis:
Question 16. Describe the etiology, epidemiology, pathogenesis, clinical features, and treatment of viral hepatitis.
(or)
Write short note on the diagnosis, prevention, and management of acute viral hepatitis.
(or)
List the viruses causing acute hepatitis.
Answer:
Viral Hepatitis Etiology:
Various causes of acute hepatitis and types and causes of viral hepatitis:
Causes of acute hepatitis:
- Viral hepatitis
- Other infections: Leptospirosis, malaria, dengue, brucellosis
- Alcohol
- Drugs: Paracetamol, isoniazid (INH), rifampicin, halothane
- Ischemic and vascular:
- Cardiogenic shock
- Hypotension
- Cocaine, methamphetamine, and ephedrine
- Acute Budd–Chiari syndrome
- Toxins: Amanita, carbon tetrachloride, and yellow phosphorous
- Pregnancy-related:
- Preeclampsia
- Acute fatty liver of pregnancy
- HELLP syndrome
- Immunological: Autoimmune hepatitis, primary sclerosing cholangitis
- Metabolic or hereditary:
- Nonalcoholic fatty liver disease
- Hemochromatosis
- Wilson’s disease
Types of viral hepatitis:
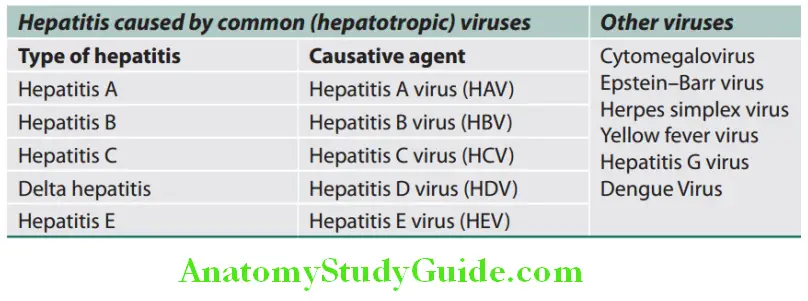
Hepatitis A:
Question 17. Discuss the clinical features, investigations, and management of hepatitis A infection.
Answer:
Hepatitis A Etiology:
- Caused by hepatitis A virus (HAV) which is a nonenveloped, 27-nm, RNA virus belonging to the Picornavirus group.
- Hepatitis A is the most common type of viral hepatitis, often occurs in epidemics.
- It most commonly affects children and young adults.
- Overcrowding and poor sanitation facilitate the spread.
Source of Infection:
- The only source of infection is acutely infected person.
- Virus replicates in the liver, is excreted in bile and then excreted in stool/feces of infected persons for about 2 weeks before the onset of symptoms and then for a further 2 weeks or so.
Mode of Spread:
Fecal-oral route (either via person-to-person contact or consumption of contaminated food or water). In outbreaks, it spreads through water, milk, and shell fish.
Incubation Period:
- 15–45 days (average 28 days).
- There is no carrier state.
- Clinical features are discussed together.
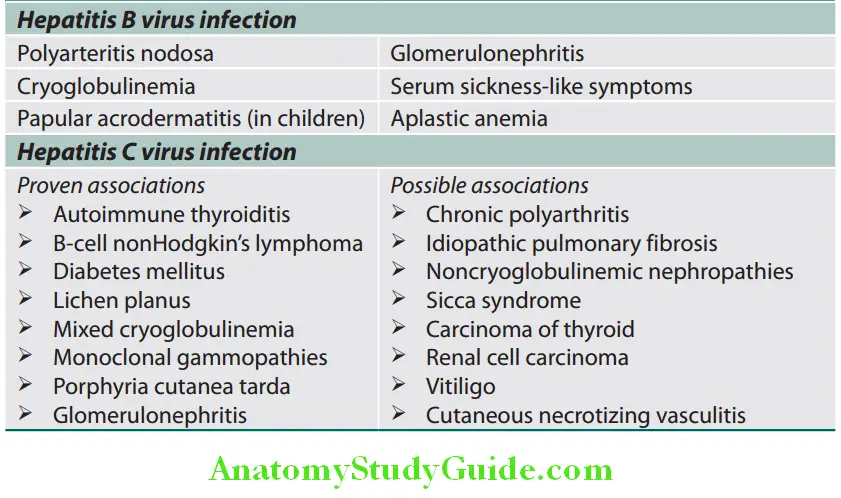
Extrahepatic Manifestations:
Extrahepatic manifestations are less frequent in acute HAV infection than in acute hepatitis B virus (HBV) infection.
Most common manifestations are quickly fading rash (14%) and arthralgias (11%) while uncommon are myocarditis, thrombocytopenia, aplastic anemia, red cell aplasia, leukocytoclastic vasculitis, glomerulonephritis, and arthritis, in which immune-complex disease is believed to play a pathogenic role.
Prevention and Prophylaxis:
- Maintain good hygiene and improve social conditions. HAV is resistant to chlorination but is killed by boiling water for 10 minutes.
- Active immunization: A formaldehyde-inactivated HAV vaccine (contains the single HAV antigen) for active immunization and can be used in individuals above the age of 2 years. It probably provides lifelong immunity.
- Passive immunization: Normal human immunoglobulin [0.02 mL/kg intramuscularly (IM)] made from pooled human plasma is used if exposure to HAV is <2 weeks and can protect from HAV infection for 3 months. HAV vaccine should also be administered.
Hepatitis B:
Question 18. What are the common causes of viral hepatitis? Discuss the clinical features, complications and management of hepatitis B infection.
Answer:
Hepatitis B Etiology:
Hepatitis B is caused by HBV which is a hepatotropic DNA virus belonging to the family Hepadnaviridae.
Structure and Genome of HBV:
Complete infective virion (HBV virion) is called as Dane particle. It is spherical 42-nm particle and double-layered comprising an inner core or nucleocapsid (27 nm) surrounded by an outer envelope of surface protein (HBsAg).
Viral genome: It consists of partially double-stranded circular DNA and has four genes.
- HBsAg (hepatitis B surface antigen) (S gene): HBsAg is a product of S gene which is secreted into the blood in large amounts. HBsAg is immunogenic.
- HBcAg (hepatitis B core antigen) (C gene): The C gene produces two antigenically different products:
- Hepatitis B core antigen (HBcAg): It remains intracellular within the hepatocytes and does not circulate in the serum. Hence, it is not detectable in the serum of patients.
- (HBeAg) (hepatitis B e antigen): It is secreted into serum and is a surrogate (substitute) marker for high levels of viral replication. It is essential for the establishment of persistent infection.
- HBV polymerase (P gene): A polymerase (Pol) is a product of P gene and DNA polymerase enzyme is needed for virus replication.
- HBxAg (X gene): HBx protein is necessary for virus infectivity and has been implicated in the pathogenesis of liver cancer in HBV infection.
Source of infection: Human suffering from hepatitis (acute/chronic) or carriers is the only source of infection. HBV is100 times as infectious as human immunodeficiency virus (HIV) and 10 times as infectious as hepatitis C virus (HCV).
Mode of Transmission:
Question 19. Write short note on mode of transmission of hepatitis B (serum hepatitis).
Answer:
- Vertical/congenital transmission: Transmission from mother [who is carrier for HBV (90% HBeAg+ve, 30% HBeAg–ve)] to child may occur in utero, during parturition, or soon after birth. It not transmitted by breastfeeding.
- Horizontal transmission: It is the dominant mode of transmission.
- Parenteral: It is the major route of transmission but occasionally nonparenteral.
- By percutaneous and mucus membrane exposure to infectious body fluids, through minor cuts/abrasions in the skin or mucus membranes. In children, it can be transmitted through minor abrasions or close contact with other children. HBV can survive for long periods on household articles, e.g., toys, toothbrushes, and may transmit the infection.
- Intravenous route: HBV can transmitted through transfusion of unscreened infected blood or blood products. This mode of spread is rare now, because of routine screening of all blood donors for HBV and HCV. Intravenous drug abuse with sharing of needles and syringes, tattooing, and acupuncture are other ways of developing infection.
- Close personal contact: Nonparenteral route of transmission includes spread through body fluids (virus can be found in these fluids) such as saliva, urine, semen, and vaginal secretions. However, this requires close personal contact, unprotected heterosexual or homosexual intercourse.
- Parenteral: It is the major route of transmission but occasionally nonparenteral.
Incubation period: 30–180 days (mean, 8–12 weeks) Chronic carrier state can develop with HBV infection (1–20%).
Prevention and Prophylaxis:
Question 20. Write short on prevention and prophylaxis of HBV.
Answer:
- Avoiding risk factors:
- Not to sharing of needles
- Having safe sex
- Transfuse safe blood and blood products
- Enforced strict standard safety precautions in laboratories and hospitals to avoid accidental needle punctures and contact with infected body fluids
- Active immunization: By using recombinant vaccines (containing HBsAg). It is advised in following individuals:
- Children: In India, nonpercutaneous routes of transmission are quite prevalent and active immunization using vaccine is recommended in all children.
- High-risk groups: For example, healthcare personnel, hemodialysis patients, injection drug users, hemophiliacs and sexual contacts of HBsAg carriers.
- Dosage regimen: Three injections are given into the deltoid muscle at 0, 1, and 6 months. Dose is 10 µg for children under 10 years and 20 µg in children above 10 years. More frequent and larger doses are required in individuals over 50 years of age or clinically ill and/or immunocompromised (including HIV infection or AIDS). Side-effects are very few.
- Combined prophylaxis: This consists of vaccination and immunoglobulin. It should be given to individuals with:
- Accidental needle-stick injury, gross personal contamination with infected blood and exposure to infected blood in the presence of cuts and grazes
- All newborn babies of HBsAg-positive mothers
- Regular sexual partners of HBsAg-positive patients, who have been found to be HBV negative
- Dosage: For adults, a dose 0.05–0.07 mL/kg bodyweight hepatitis B immunoglobulin (HBIG) (200 IU to newborns) and the vaccine (IM) given at another site.
Mode of Transmission Treatment: Pegylated interferon alpha, lamivudine, adefovir, entecavir, telbivudine, or tenofovir may be used as initial therapy but lamivudine and telbivudine are not preferred because of high rates of resistance.
Hepatitis C:
Hepatitis C Etiology:
- Previously called bloodborne non-A, non-B hepatitis.
- It is a small, enveloped, single-stranded RNA virus belonging to the family Flaviviridae.
- A characteristic feature is emergence of an endogenous, newly mutated strain. Because of genomic instability and antigenic variability, producing an effective HCV vaccine is difficult.
- HCV has six genotypes and in India, most prevalent is HCV
- Mode of spread: HCV is not transmitted by breastfeeding.
- It is mainly transmitted by the parenteral route (transfusion of blood and blood products, and in drug addicts) as a bloodborne infection
- Sexual contact (low chances of transmissio
- Perinatal/vertical transmission
- Incubation period: 15–160 days (mean, 7 weeks):
- Nearly 80% infected individuals develop chronic hepatitis.
Hepatitis D:
Question 21. Write short note/essay on etiology and epidemiology of delta hepatitis.
Answer:
Hepatitis D Etiology:
- Caused by hepatitis D virus (HDV or delta virus), which is a defective/incomplete RNA virus and belongs to the Deltaviridae family. The RNA genome is covered by an outer coat/shell of HBsAg.
- It has no independent existence and requires HBV for its replication and expression.
- Because HDV is dependent on HBV, the duration of HDV infection is determined by the duration of HBV infection. It causes delta hepatitis (Hepatitis D) with two clinical patterns.
- Acute coinfection: It develops when individual is exposed simultaneously to serum, containing both HDV and HBV. The HBV infection first becomes established and the HBsAg is necessary for development of complete HDV virions.
- Superinfection: It occurs when an individual is already infected with HBV (chronic carrier of HBV) is exposed to a
new dose HDV.
- Mode of spread: Parenteral route and sexual contact.
- Fulminant hepatitis can follow both patterns of infection but is more common after coinfection.
Hepatitis E:
Question 22. Write short note/essay on transmission, clinical features, and management of hepatitis E infection.
Answer:
It was previously called epidemic or enterically transmitted non-A, non-B hepatitis.
Hepatitis E Etiology:
- Hepatitis E virus (HEV) is an unenveloped, single-stranded RNA virus in the Hepevirus genus.
- Viral particles are 32–34 nm in diameter.
- Hepatitis E occurs primarily in young to middle-aged adults.
- Source of infection: HEV is a zoonotic disease with animal reservoirs, such as monkeys, cats, pigs, rodents, and dogs.
- Virions are shed in stool during the acute illness.
- Mode of transmission: An enterically transmitted, waterborne infection. It is common after contamination of water supplies such as after monsoon flooding.
- Incubation period: 14–60 days (mean, 5–6 weeks).
Outcome of Infection:
- HEV infection is responsible for more than 30–60% of cases of sporadic acute hepatitis (clinically very similar to hepatitis A) in India. It produces self-limiting acute hepatitis.
- It does not cause chronic liver disease.
- High mortality rate (about 20%) among pregnant women.
Prevention and Control:
- Good sanitation and hygiene similar to hepatitis A
- Vaccine has been developed and used successfully in China.
Clinical Features of Viral Hepatitis:
Question 23. Write short note/essay on clinical features of acute hepatitis/hepatitis A/hepatitis B.
Answer:
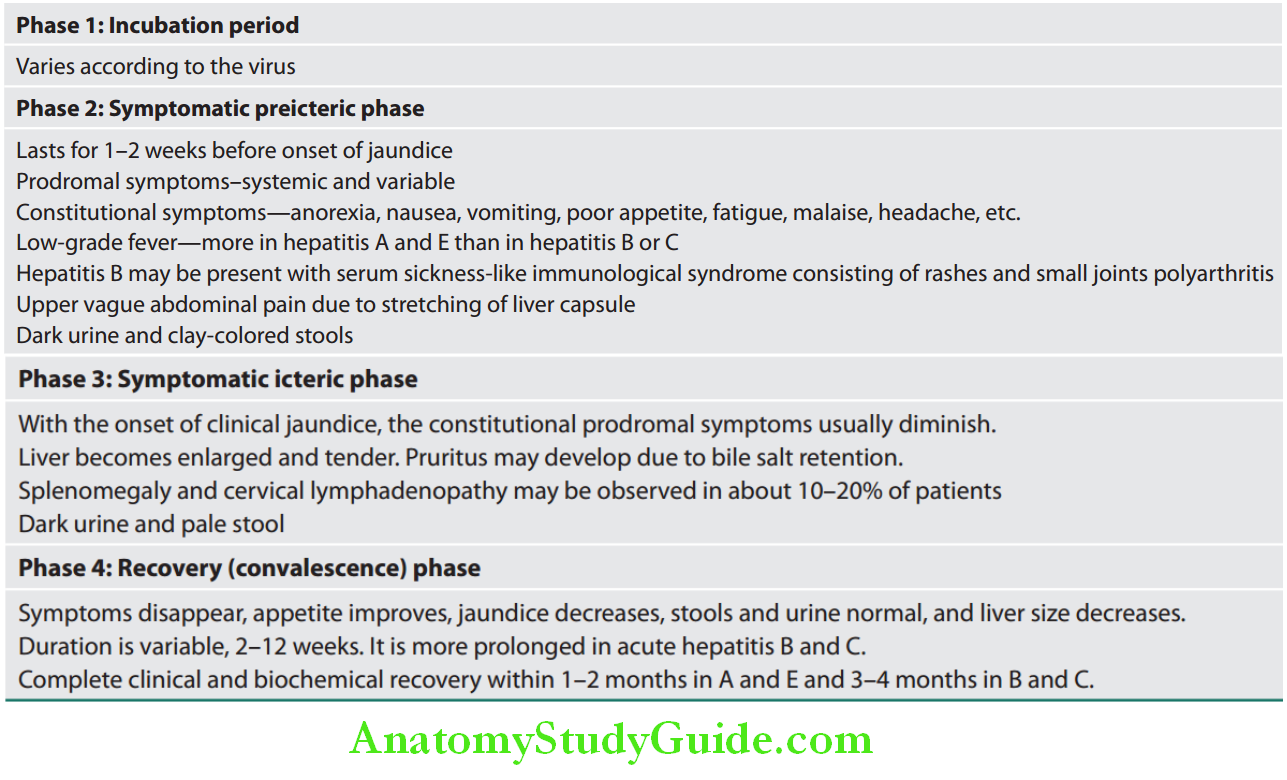
Fulminant Hepatitis:
It can develop in hepatitis B, D, and E. It is uncommon with hepatitis C and rare in hepatitis A. With hepatitis E, fulminant hepatitis occurs in nearly 20% cases in pregnant females.
Summary of various hepatotropic viruses are presented in Table:
Summary of various hepatotropic viruses:
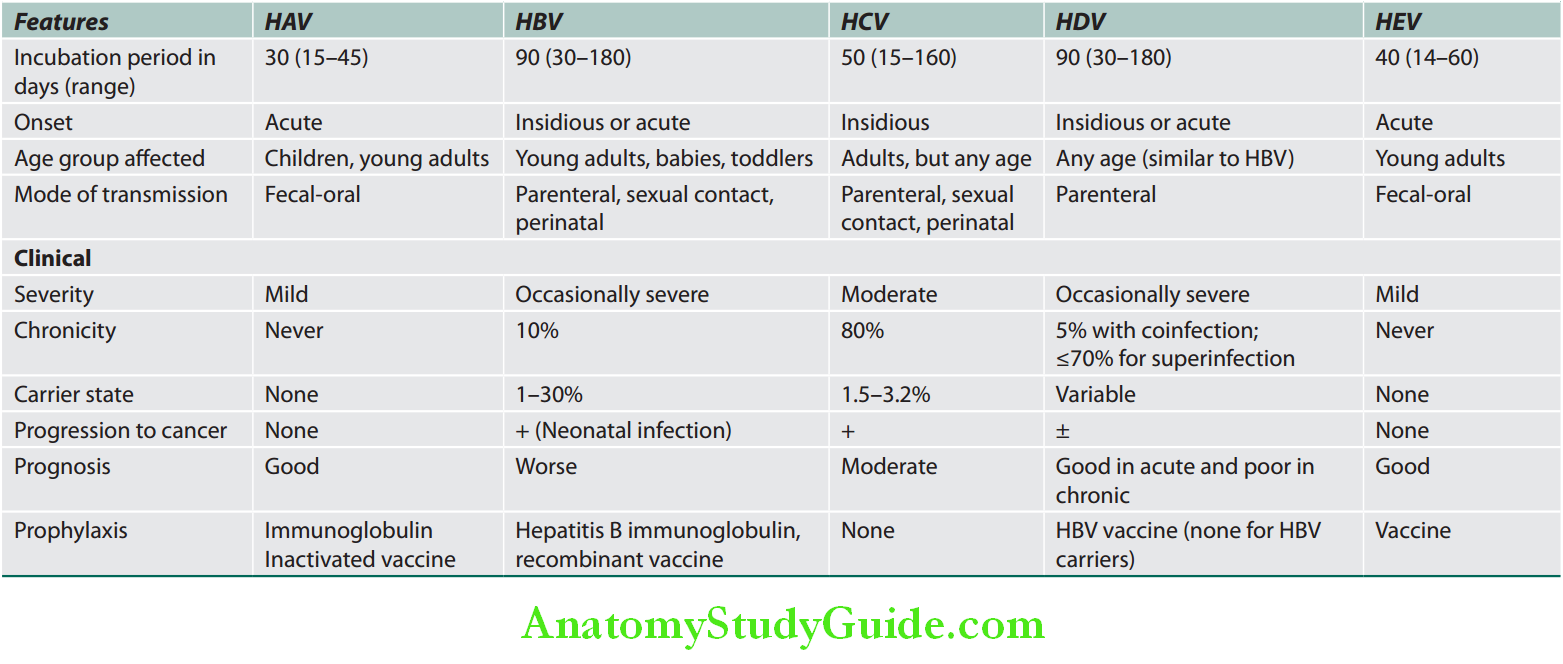
Fulminant Hepatitis Investigations:
- Urine:
- Bilirubinuria (in early stages) and increased urinary urobilinogen slight microscopic hematuria and mild proteinuria
- Hematological tests:
- Leukopenia with a relative lymphocytosis
- Prothrombin time (PT) is prolonged in severe cases which signifies extensive hepatocellular damage. This is one of the best indices of prognosis.
- Erythrocyte sedimentation rate (ESR) is raised
- Biochemical investigations
- Aminotransferases (AST, ALT): Raised and maximum levels are observed during the prodromal phase (400–4,000 IU/L). They progressively decline during icteric and recovery phases.
- Bilirubin: Both conjugated and unconjugated bilirubin levels are equally raised.
- ALP: It may be raised but usually less than two times the normal.
- Serum protein: Normal
- Blood glucose: It may below.
- Serological tests: During prodromal phase, low titers of antismooth muscle antibody, rheumatoid factor, antinuclear antibody, and heterophil antibody may be observed.
Serological Markers for Viral Hepatitis:


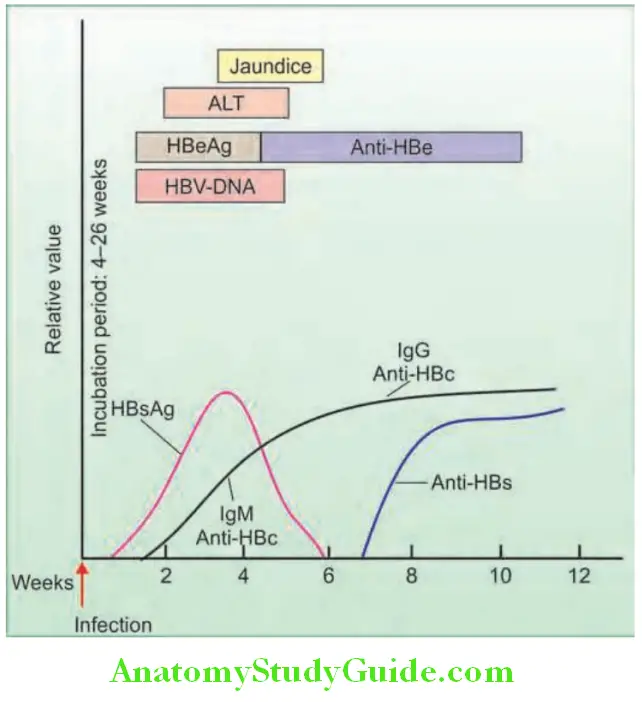
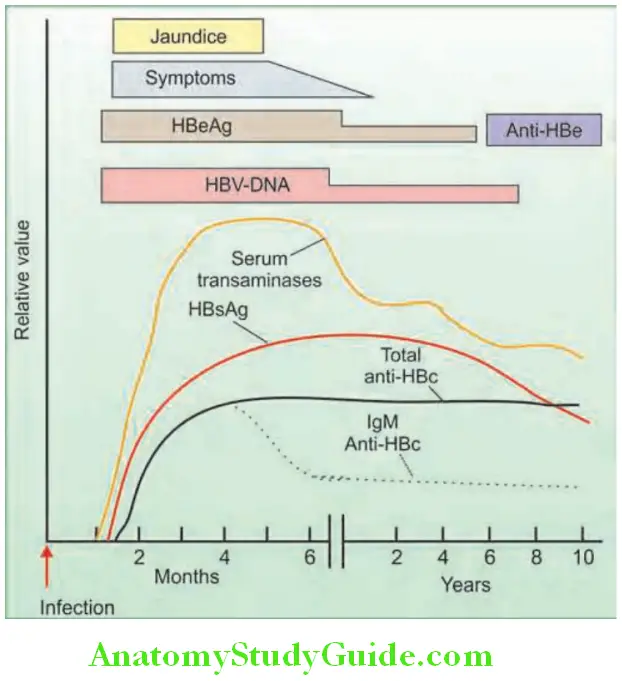
Question 24. Write short note on HBsAg/Australia antigen/hepatitis B surface antigen.
Answer:
Interpretations of serological findings and its significance in HBV are summarized in Table:
HBV viral DNA (HBV DNA): Most accurate marker of virus replication:
Interpretation of serological fidings and its signifiance in HBV:
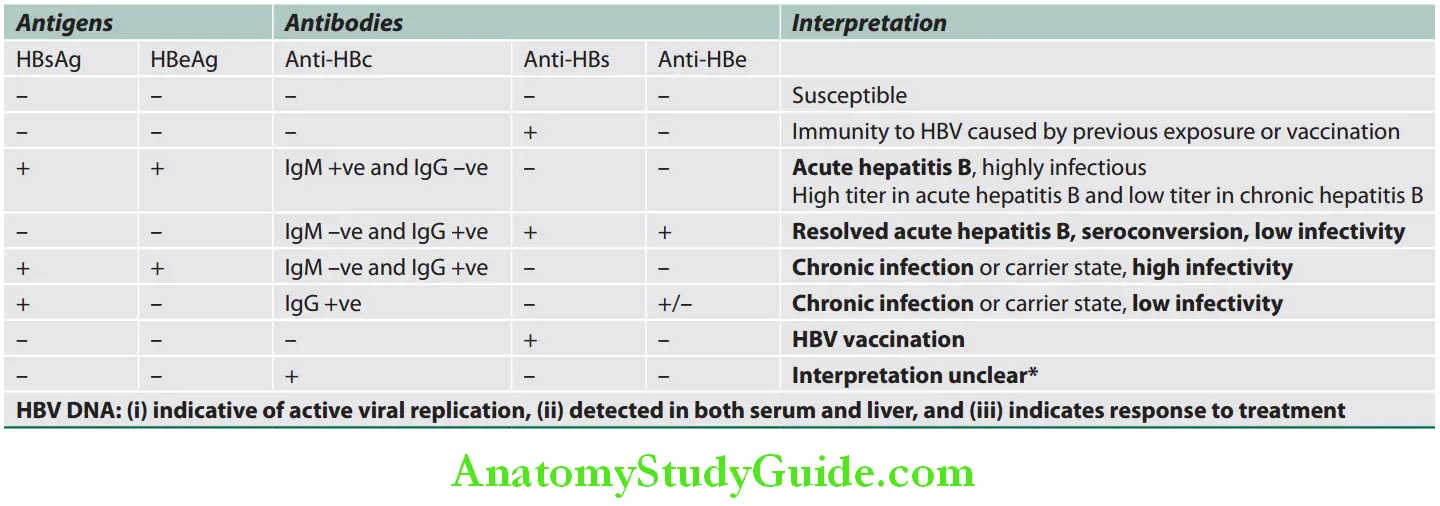
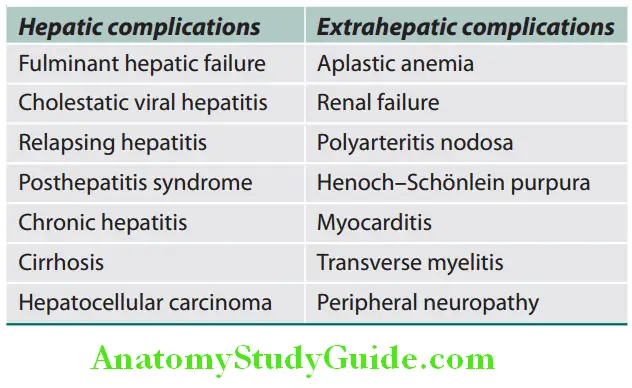
Question 25. Write short note on complications of acute viral hepatitis.
Answer:
Complications of Acute Viral Hepatitis:
Hepatitis C:
- Anti-HCV: It appears after the infection, disappears after the recovery, and persists in chronic hepatitis C.
- HCV-RNA: It appears after the exposure.
- Anti-HCV (antibodies against HCV) does not confer immunity.
Hepatitis D:
- HDV RNA: Detectable in the blood and liver before and in the early days of acute disease.
- Anti-HDV: IgM anti-HDV is the most reliable indicator of recent HDV exposure.
Hepatitis E:
- Before the onset of clinical illness, HEV RNA and HEV virions can be detected in stool and serum.
- After the onset of clinical illness, serum aminotransferases rise and elevated IgM anti-HEV titers also occur simultaneously. After recovery, the IgM is replaced with a persistent IgG anti-HEV titer.
- Poor prognostic features of viral hepatitis are listed in Table:
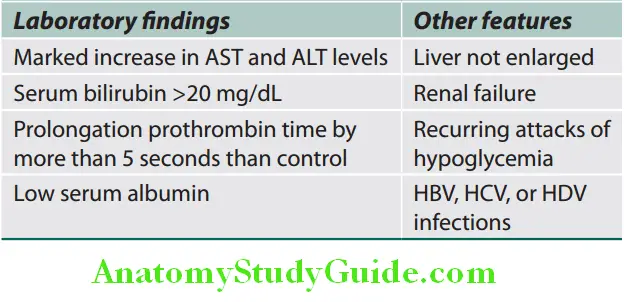
Acute Viral Hepatitis Treatment:
General Measures:
- Avoid drugs which are metabolized in the liver, e.g., sedatives and narcotics.
- Avoid alcohol during the acute illness.
- No specific dietary modifications are required.
- Elective surgery should be avoided during acute viral hepatitis, as there is a risk of postoperative liver failure.
- Liver transplantation is performed for complications of cirrhosis resulting from chronic hepatitis B and C infection.
Hepatitis A:
Question 26. Write short note on treatment of hepatitis A infection.
Answer:
- No specific treatment:
- Rest and dietary measures are not helpful.
- Supportive symptomatic treatment
- Corticosteroids do not have benefit.
Hepatitis B:
Question 27. Write short note on treatment of hepatitis B infection.
Answer:
Therapeutic goal:
- It prevents the progression to end-stage liver disease, hepatocellular carcinoma (HCC), and death with improvement in quality of life.
- Clearance of HBV DNA
- Absence of HBeAg and HBsAg and appearance of antibody
- Normalization of liver enzymes and histology
Acute Hepatitis B:
- In previously healthy adults, recovery occurs in ~99%; therefore, antiviral therapy is not required.
- Mainly symptomatic.
- Monitor HBV markers.
- Entecavir (ETV) or tenofovir (TDF) to be given when HBeAg persists beyond 12 weeks, and in patients who are very ill.
Postexposure Prophylaxis to Prevent Hepatitis B Virus Infection:
Hepatitis C:
- Goals of treatment of HCV:
- Eradication Of Virus,
- Reduce Progression Of Disease,
- Histological Improvement
- Decrease frequency of HCC.
- Interferon-∝ is used in acute hepatitis C to prevent chronic disease.
- HCV: Progression of fibrosis determines the prognosis and liver biopsy is the gold standard to assess fibrosis.
Hepatitis D:
Active liver disease (raised ALT levels and/or inflammation on biopsy) is treated with peginterferon ∝-2a and adefovir for 12 months.
Transfusion-associated Hepatitis (TAH):
- It includes HCV and/or HBV (discussed above).
Chronic Hepatitis:
Question 28. Discuss the classification, etiology, pathology, clinical features, complications, prevention, and management of chronic hepatitis/chronic hepatitis B infection.
(or)
Write short essay on chronic active hepatitis.
Answer:
Chronic Hepatitis Definition:
Chronic hepatitis is defined as symptomatic, biochemical, or serologic evidence of hepatic disease for more than 6 months. Microscopically, there should be inflammation and necrosis in the liver.
Chronic Hepatitis Classification:
Older classification: Previously chronic hepatitis was classified into:
- Milder forms:
- Chronic persistent hepatitis (CPH)
- chronic lobular hepatitis (CLH)
- Severe forms: Chronic active hepatitis (CAH)
However, this classification is not very helpful in determining the prognosis.
Present classification: In order to assess response to therapy and prognosis, present classification is based on combination of three factors.
Basis of present classification of chronic hepatitis:
- Etiology: Cause of hepatitis
- Grade: Histologic activity
- Stage: Degree of progression
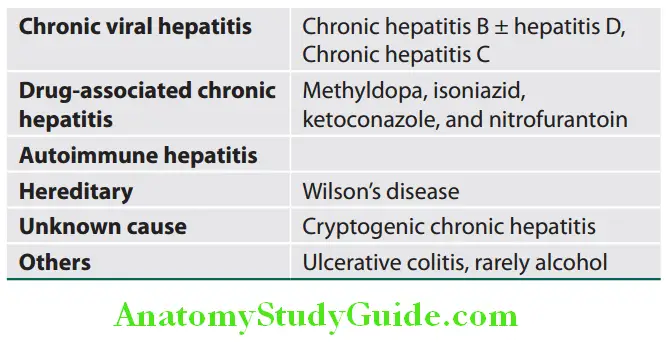
Classification Based on the Causes of Chronic Hepatitis:
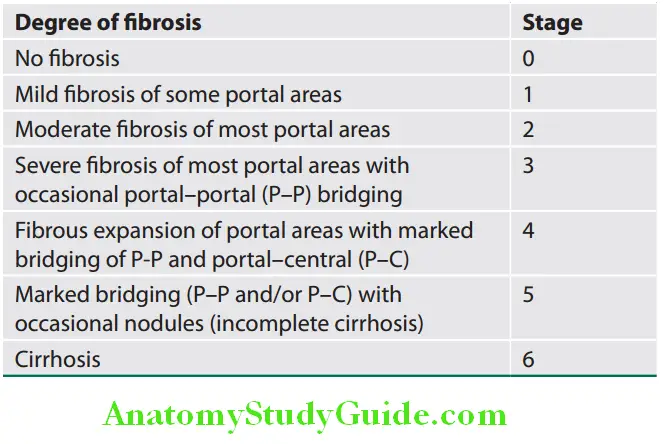
It indicates level of progression and based on the degree of hepatic fibrosis
Autoimmune Hepatitis:
Question 29. Write short note on clinical features and treatment of autoimmune hepatitis.
Answer:
Autoimmune hepatitis (AIH) is a chronic and progressive (unresolving) hepatitis of unknown cause. No features are absolutely diagnostic and are associated with circulating autoantibodies and hypergammaglobulinemia.
Autoimmune Hepatitis Clinical Features:
- It may be asymptomatic or present with fatigue (most common), anorexia, jaundice, myalgia, and diarrhea.
- Acute hepatitis: In about 30% of cases, it may present as acute hepatitis similar to viral hepatitis, which does not resolve with time.
- Fulminant hepatic failure (FHF) or asymptomatic elevation of serum ALT.
- Cirrhosis: In about 25% patients.
- Jaundice may be mild to moderate and is found in 69% of patients.
Autoimmune Hepatitis Investigations:
Biochemical findings:
- Serum aminotransferases: High and more than 10 times during relapses
- Serum bilirubin: Mildly raised usually less than 6 mg/dL
- Serum ALP: Mildly raised
- Serum γ-globulins: High; hypergammaglobulinemia is polyclonal; the IgG fraction predominates.
- Serum albumin: Low
- Serum al-antitrypsin, serum ceruloplasmin, iron and ferritin levels: Normal.
Autoantibodies:
Classification of autoimmune hepatitis based on immunological markers and autoantibodies is presented.
Classification of autoimmune hepatitis based on immunological markers:
Based on the differences in immunological markers AIH is divided into:
- Type 1 AIH is characterized by anti-smooth muscle antibodies (SMAs) and antinuclear antibodies (ANA), or both. Most commonly seen in females and associated with extrahepatic immunologic diseases such as autoimmune thyroiditis, Graves’ disease, ulcerative colitis, rheumatoid arthritis, and Coombs-positive hemolytic anemia.
- Type 2 AIH is characterized by antibodies to liver/ kidney microsome type 1 (anti-LKM1). Most affected persons are children.
- Type 3 AIH: Presence of antibodies to soluble liver antigen/liver pancreas (anti-SLP/LP)
Autoantibodies Other tests:
- HBsAg and other viral markers: Negative
- Prothrombin time: Prolonged
Liver biopsy:
Chronic hepatitis, variable amounts of interface hepatitis, Cirrhosis, bridging necrosis
Autoantibodies Treatment:
- Prednisolone: 30 mg given orally daily for 2 weeks. Gradually tapering dose as LFT improves. Maintenance dose of 10–15 mg daily for at least 2 years after LFT has become normal.
- Azathioprine: Dose of 1–2 mg/kg daily, added as a steroid-sparing agent and some patients for sole long-term maintenance therapy or if dose of prednisolone is more than 10 mg/day.
- Other immunosuppressive agents: Mycophenolate, ciclosporin and tacrolimus for resistant cases.
- Duration of treatment: Lifelong in most cases
- Liver transplantation: If treatment fails
Course and Prognosis:
- Exacerbations and remissions are common. Progression of steroid and azathioprine therapy produces remission in over 80% of patients.
- It may progress to hepatic failure and death.
- Some may develop cirrhosis and its complications.
- Hepatocellular carcinoma is uncommon.
Chronic Hepatitis B:
Question 30. Write short note on complications of HBV infection.
Answer:
- Chronic HBV infection occurs following an acute HBV infection (may be subclinical) and occurs in about 1–10% of patients.
- HBV infection is considered as chronic when the HBsAg (hepatitis B surface antigen) persists for more than 6 months.
- It may progress to cirrhosis and HCC.
- Risk of chronic hepatitis Depends on:
- Age at which the contact of acute infection: Chronic hepatitis occurs more commonly with neonatal (90%) or childhood (20–50% below the age of 5 years) infection rather than in adult life (<10%).
- Immune status: In immunocompetent adults, the incidence of acute hepatitis is high while chronic infection is rare (1–2% of cases).
Other conditions where incidence of chronic hepatitis B infection is high are given in Table:
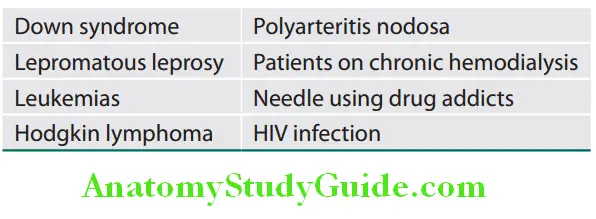
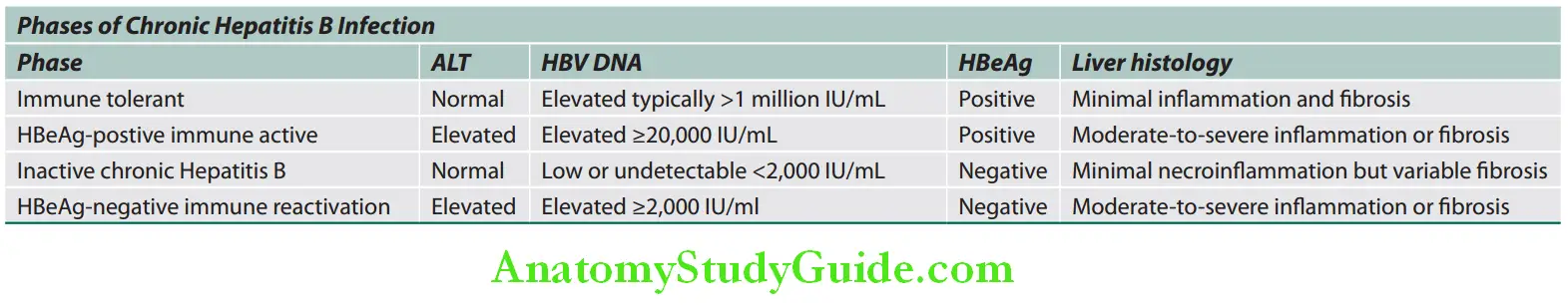
Phases of Chronic HBV Infection:
1. Immune-tolerant phase Characteristic features are:
- Asymptomatic and frequent in children. Infection during birth or in early childhood develop prolonged immune-tolerant phase, and disease progresses even after the disappearance of HBeAg in some of these patients.
- Active viral replication in liver but little or no evidence of disease activity.
- HBsAg and HBeAg positive and very high levels of serum HBV DNA.
- Normal LFTs
- Liver biopsy shows no inflammation or fibrosis.
- It may lasts for decades. Therefore, lifelong monitoring is necessary.
2. Immune-active phase (chronic hepatitis): Most patients with immune-tolerant phase progress to the immune-active phase.
- Vigorous immune response.
- Criteria for chronic HBV active hepatitis are:
- Liver biopsy shows chronic hepatitis with moderate or severe necroinflammation and fibrosis.
- Evidence of active HBV replication: High levels of HBV DNA and HBeAg
- Persistent or intermittent elevation of serum aminotransferases (ALT/AST)
3. Inactive chronic Hepatitis B—carrier phase with low replication: Incidence varies. Most patients with chronic HBV infection will eventually enter inactive carrier phase.
- Criteria for carrier phase:
- Serological findings:
- HBsAg positive in the serum > 6 months.
- HBeAg negative and HBe antibody positive (seroconversion from HBeAg to HBeAb).
- Undetectable or low levels (below 400 IU/L) of HBV DNA in the serum.
- Normal aminotransferase (ALT) levels.
- Liver biopsy does not show any significant hepatitis.
- Low-risk for hepatocellular carcinoma
- Liver abnormalities generally do not progress to more severe disease.
- Disease may be reactivated by severe immunosuppression (e.g., during chemotherapy for cancer or with bone marrow transplantation).
Individuals infected during adults or adolescence usually become inactive carriers after they clear HBeAg.
4. Chronic HBeAg-negative immune reactivation:
- Patients harbor HBV variants with mutations that prevent production or have low HBeAg.
- HBV DNA levels are high, liver enzymes are raised, and active histological activity is present.
- Late phase in the natural history of chronic HBV and often seen in older patients with advanced disease
HBV genotype C infection (prevalent in India) has an increased chance of developing cirrhosis and HCC.
Chronic HBV Infection Clinical Features:
- Asymptomatic or may develop severe end-stage liver disease.
- Symptoms: Fatigue, malaise, and anorexia, persistent or intermittent jaundice.
- During end-stage liver disease: Symptoms due to complications of cirrhosis occur.
- Extrahepatic manifestations: Arthralgias, arthritis, vasculitis, glomerulonephritis, and polyarthritis nodosa.
- Mild hepatomegaly
- Long-standing cases may develop HCC.
Chronic HBV Infection Investigations:
Biochemical investigations:
- Serum aminotransferase: Mildly elevated but may be as high as 1,000 units. ALT (SGPT) tends to be more raised than
compared to AST (SGOT). Once cirrhosis develops, AST levels exceeds ALT. - Serum bilirubin: It may be normal or raised up to 10 mg/dL.
- Serum proteins: Hypoalbuminemia in severe cases and hyperglobulinemia
Prothrombin time: Prolonged
Serological markers:
Serological markers of chronic hepatitis B:
- Positive HBsAg
- Positive IgG anti-HBc, negative IgM anti-HBc
- Positive HBe antigen or rarely, positive anti-HBe
- Positive HBV DNA
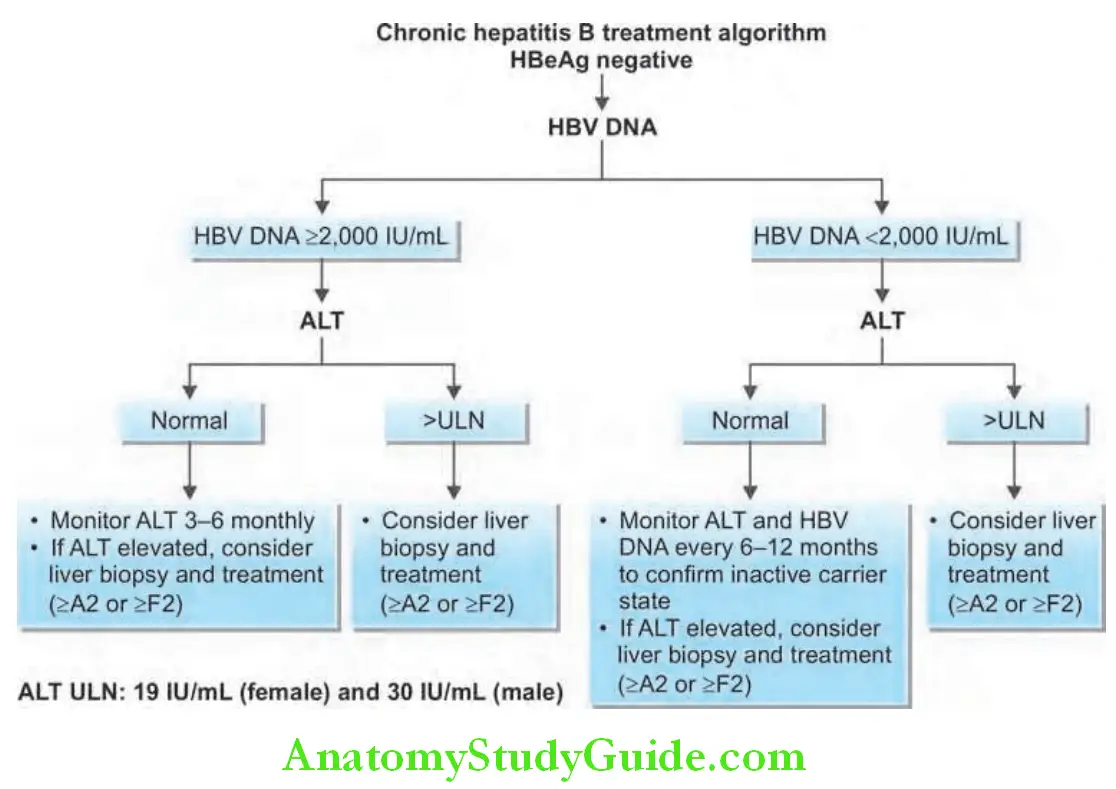
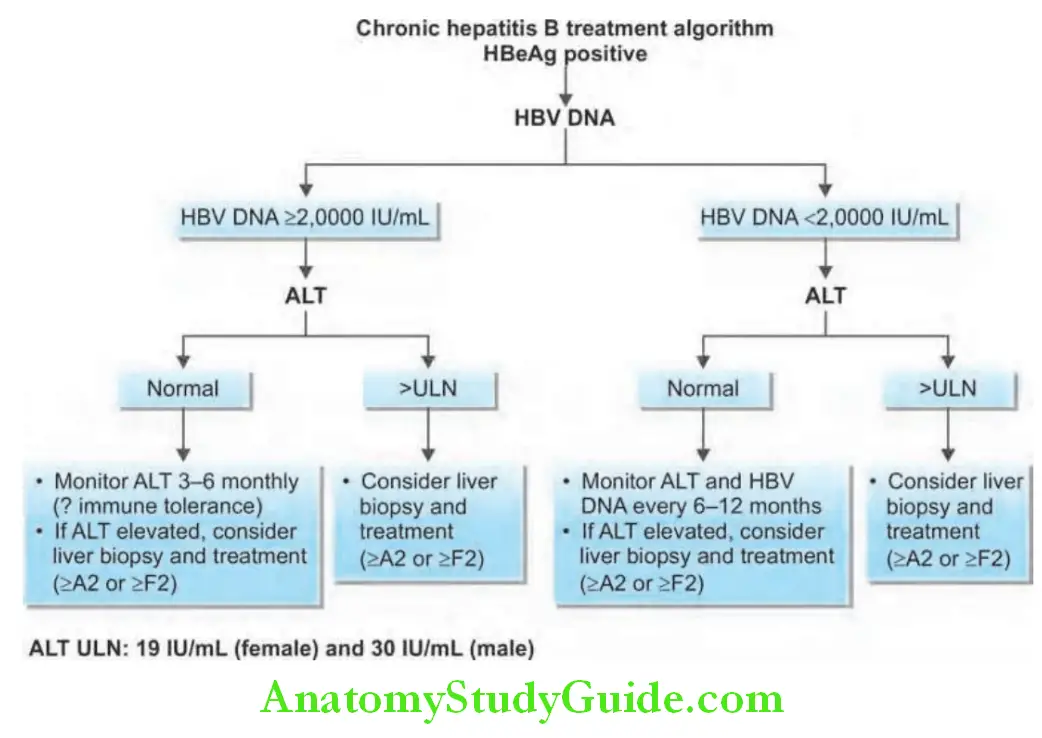
Treatment for Chronic Hepatitis B:
Criteria: Three criteria are used namely: serum levels of HBV DNA, serum levels of ALT, and histological grade and stage.
- Serum HBV DNA above 2000 IU/mL (about >10,000 copies/mL)
- Serum ALT level greater than two times the normal
- Moderate-to-severe active necroinflammation and/or fibrosis in the liver biopsy
In presence of cirrhosis (compensated or decompensated), oral antiviral agents are recommended but liver transplantation may be necessary.
Patients without cirrhosis: Pegylated interferon, tenofovir, and entecavir are the preferred agents in patients with clinically compensated cirrhosis, entecavir or tenofovir (tenofovir alafenamide or tenofovir disoproxil fumarate) is preferred.
For patients with decompensated cirrhosis, interferon is contraindicated, and either entecavir or tenofovir should be used.
Immunotolerant patients, usually young with normal ALT and high HBV DNA levels, without evidence of liver disease do not need therapy, but be regularly followed-up.
Aim of treatment:
Aim of treatment of chronic hepatitis B:
- Seroconversion of HBeAg when present to anti-HBe. When HBeAg disappears, remission is usually attained for several years.
- Reduction of HBV DNA to 400 IU/L or less.
- Achieve normal levels of serum ALT.
- Histological improvement in inflammation and fibrosis in the liver biopsy
- Patients usually remain HBsAg positive, but loss of serum HBsAg indicates a good response.
Antiviral agents:
Most commonly used drugs are:
Drugs used for chronic hepatitis B:
- Peginterferon in combination with other agents:
- Lamivudine plus peginterferon
- Entecavir plus peginterferon
- Tenofovir plus peginterferon
- Adefovir plus peginterferon
- Telbivudine plus peginterferon
- Lamivudine plus adefovir dipivoxil
- Tenofovir disoproxil plus entecavir
- Tenofovir disoproxil plus emtricitabine
Pegylated α-2a interferon:
- Response (defined as loss of HBeAg and HBV DNA) occurs in 25–40% of cases.
- Dose: 180 μg once a week subcutaneously and produces response after 48 weeks of treatment.
- Side effects: Acute flu-like symptoms, malaise, headache, depression, reversible hair loss, bone marrow depression, thrombocytopenia, and infection
- Patients with HIV respond poorly and it should not be given to patient with cirrhosis.
Entecavir:
- A cyclopentyl guanosine analog that is a very effective and quickly reduces HBV DNA by 48 weeks.
- 0.5–1 mg daily
Tenofovir:
- It is a cytosine nucleoside analog which is also very effective and has a similar potency to entecavir. It is used for HIV patients with HBV infection.
- Tenofovir alafenamide (25 mg daily) OR tenofovir disoproxil fumarate (300 mg daily)
Lamivudine:
- It is well tolerated. However, rate of development of viral resistance (80%) is high and itself may cause hepatitis. Hence, lamivudine monotherapy is no longer recommended.
- Dosage is 100 mg/day given orally once a day until HBeAg becomes negative.
- Used if coinfection with HIV
Adefovir dipivoxil:
- A nucleotide reverse transcriptase inhibitor.
- 10 mg daily is used in treatment of patients with lamivudine-resistant HBV
Telbivudine:
- An L-nucleoside that may cause myopathy and neuropathy.
- The recommended dose is 600 mg once daily
Chronic HBV Infection Prognosis:
- Development of chronic hepatitis depends on the age at which infection is acquired.
- Development of cirrhosis is associated with a poor prognosis.
- Hepatocellular carcinoma is one of the most common carcinomas in HBV-endemic areas.
Chronic Hepatitis C:
Question 31. Write short note on complications of HCV.
Answer:
- Chronic hepatitis occurs in the majority (70–85%) of individuals infected by HCV and is the hallmark of HCV infection.
- Cirrhosis develops over 5–20 years in 20–30% of patients, while HCC also develops in several patients, especially with cirrhosis.
- Factors that accelerate progression to advanced liver disease includes: Alcohol consumption, coinfection with HIV or HBV, and older age at the time of acquiring the infection.
Chronic Hepatitis C Clinical Features:
- Usually asymptomatic. Detected following a routine biochemical test when mild elevations in the aminotransferases (usually ALT) are detected.
- Clinical features when present are similar to chronic hepatitis B. Most common being fatigue and jaundice being rare.
- Extrahepatic features.
Chronic Hepatitis C Investigations:
- HCV antibody in the serum is detected in more than 95% cases.
- HCV RNA detectable in all patients
- Liver biopsy is performed if active treatment is being considered. The histological changes are highly variable. It most commonly shows features of chronic hepatitis, often with lymphoid follicles in the portal tracts, and fatty change.
- Other laboratory features are similar to those seen in chronic hepatitis B.
Extrahepatic Manifestations of Hepatitis B and Hepatitis C Virus Infection:
Extrahepatic manifestations of B and hepatitis C virus infection:
Treatment of Chronic Hepatitis C:
- Indications for treatment:
- Chronic hepatitis on liver histology with HCV RNA in the serum and raised serum aminotransferases for more than 6 months.
- Chronic hepatitis with persistently normal aminotransferases.
- Cirrhosis, fibrosis, or moderate inflammation on liver biopsy (biopsy not mandatory).
- The goal of antiviral therapy in patients with chronic hepatitis C virus (HCV) is to eradicate HCV RNA, which is predicted by attainment of a sustained virologic response (SVR), defined as an undetectable RNA level 12 weeks following the completion of therapy.
- Liver transplant: For patients with decompensated cirrhosis.
Drugs for the treatment of chronic hepatitis C:

Antivirals for HCV infection in treatment-naive patients:
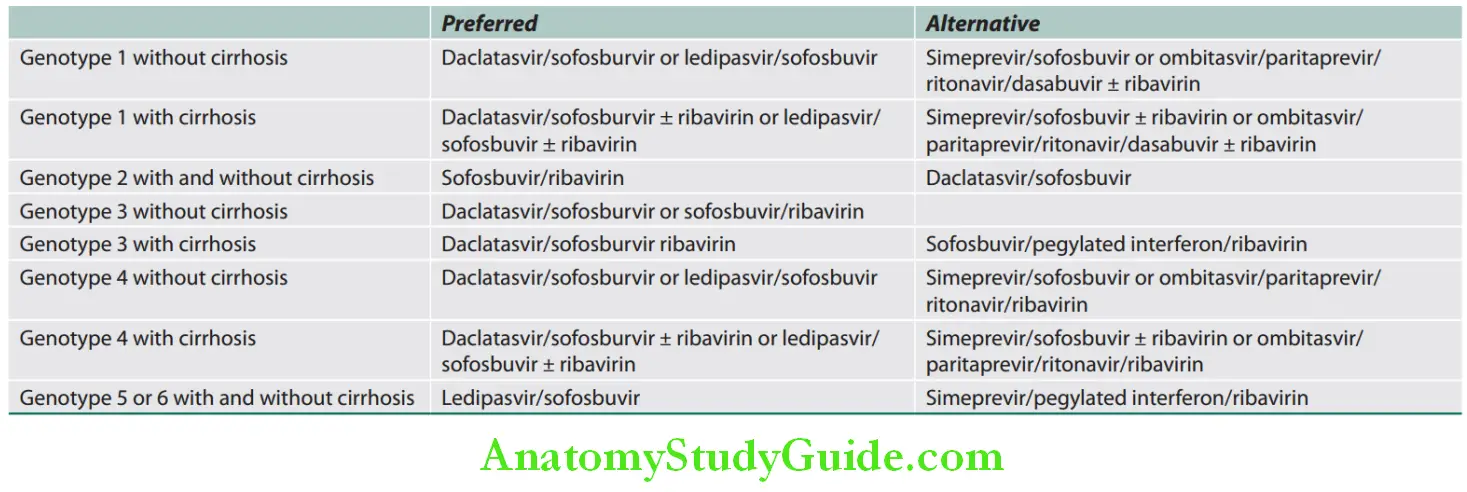
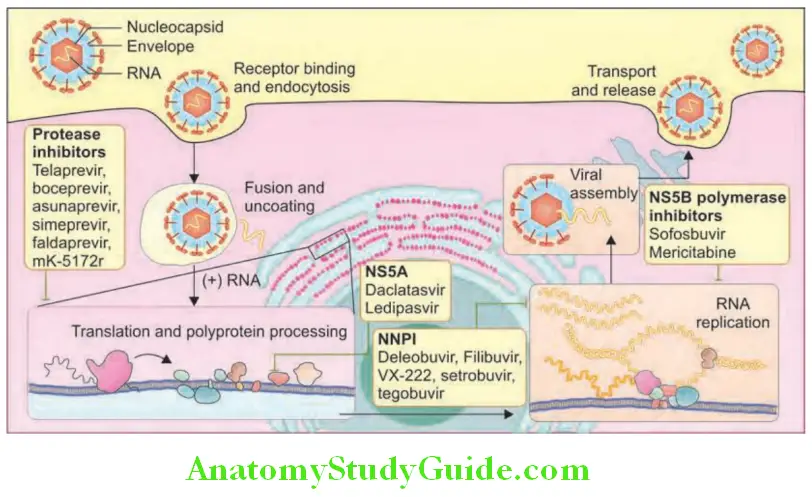
Acute Liver Failure:
Question 32. Define fulminant hepatic failure. Discuss the causes, pathology, clinical features, investigations, complications, and management of fulminant hepatic failure.
(or)
Enumerate the precipitating causes of hepatic coma in a case of chronic liver disease. Discuss the diagnosis and treatment of hepatic coma.
Answer:
Acute Liver Failure Definition:
Acute liver failure is defined as the rapid progressive deterioration in the liver function, specifically coagulopathy [elevated prothrombin time/international normalized ratio (INR)] and mental status changes (encephalopathy) in a patient without known prior liver disease.
Classifiation of Acute Liver Failure:
Acute liver failure is subclassified into hyperacute, acute, and subacute, depending on the interval between onset of jaundice and encephalopathy.
- Hyperacute hepatic failure: If encephalopathy develops within 7 days, it is called hyperacute hepatic failure and has better prognosis than acute hepatic failure.
- Acute/FHF: In this, encephalopathy develops within 3 weeks (7–21 days) from onset of symptoms in a patient with a previously normal liver.
- Subacute hepatic failure: If hepatic failure develops at a slower pace (>21 days and <26 weeks), it is called subacute or subfulminant hepatic failure.
Fulminant Hepatic Failure:
It is defined as severe hepatic failure (insufficiency) in which encephalopathy develops within 8 weeks from onset of symptoms in a patient with a previously normal liver.
Acute Liver Failure Etiology: FHF is a rare but often life-threatening condition and the various causes of FHF are listed in Table
Important causes of fulminant hepatic failure:
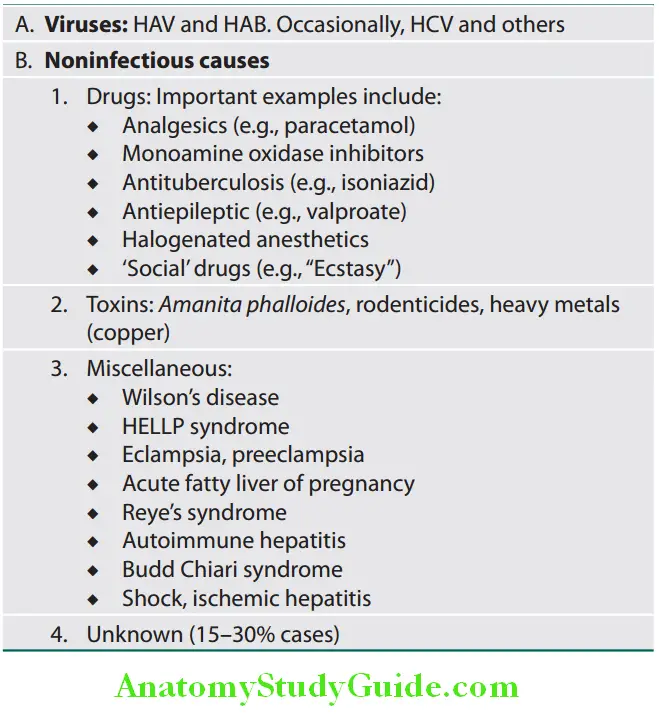
Acute Liver Failure Clinical Features:
- General features:
- Jaundice, weakness, nausea, and vomiting
- Pain in the right hypochondrium
- Small liver and liver dullness are absent on percussion.
- Ascites and edema develop later.
- Features of hepatic encephalopathy:
- Mental state: It varies from mild drowsiness, confusion, and disorientation (grades I and II) to unresponsive coma (grade IV) with convulsions.
- Fetor hepaticus and flapping tremor (asterixis) is common.
- Ascites and splenomegaly are rare.
- Fever, vomiting, hypotension and hypoglycemia may be observed.
- Spasticity and extension of the arms and legs and plantar responses remain flexor until late.
- Features of cerebral edema: Cerebral edema develops in ~80% of patients.
- Bradycardia, intracranial hypertension, and irregular respiration (Cushing’s triad).
- Pupils: Unequal or abnormally reacting or fixed pupils
- Hyperventilation and hyperreflexia.
- Consequences: Intracranial hypertension and brain herniation are the most common causes of death.
Acute Liver Failure Investigations:
Investigations to determine the cause of acute liver failure:
- Serum findings:
- Hyperbilirubinemia: Serum bilirubin is raised.
- Serum aminotransferases are raised but are not useful indicators of the course of the disease as they tend to fall with progressive liver damage.
- Coagulation factors are decreased including prothrombin and factor V. Prothrombin time is prolonged.
- Serum proteins: Hypoalbuminemia
- Plasma and urine amino acids are increased.
- Blood ammonia levels: Raised.
- Urine shows protein, bilirubin, and urobilinogen.
- Peripheral blood: Leukocytosis and thrombocytopenia.
- EEG: It may be help in grading the encephalopathy.
- Ultrasound: To detect the liver size and for any evidence of underlying liver pathology
- CSF: Intracranial pressure is raised, but CSF is normal.
Complications of FHF are listed in Table:
Complications of fulminant hepatic failure:
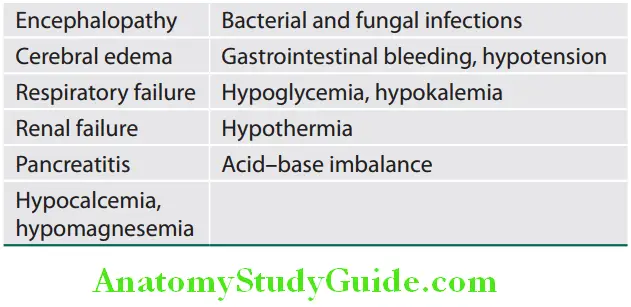
Pathogenesis and management of major complications of acute liver failure are given in Table:
Pathogenesis and management of major complications of acute liver failure:
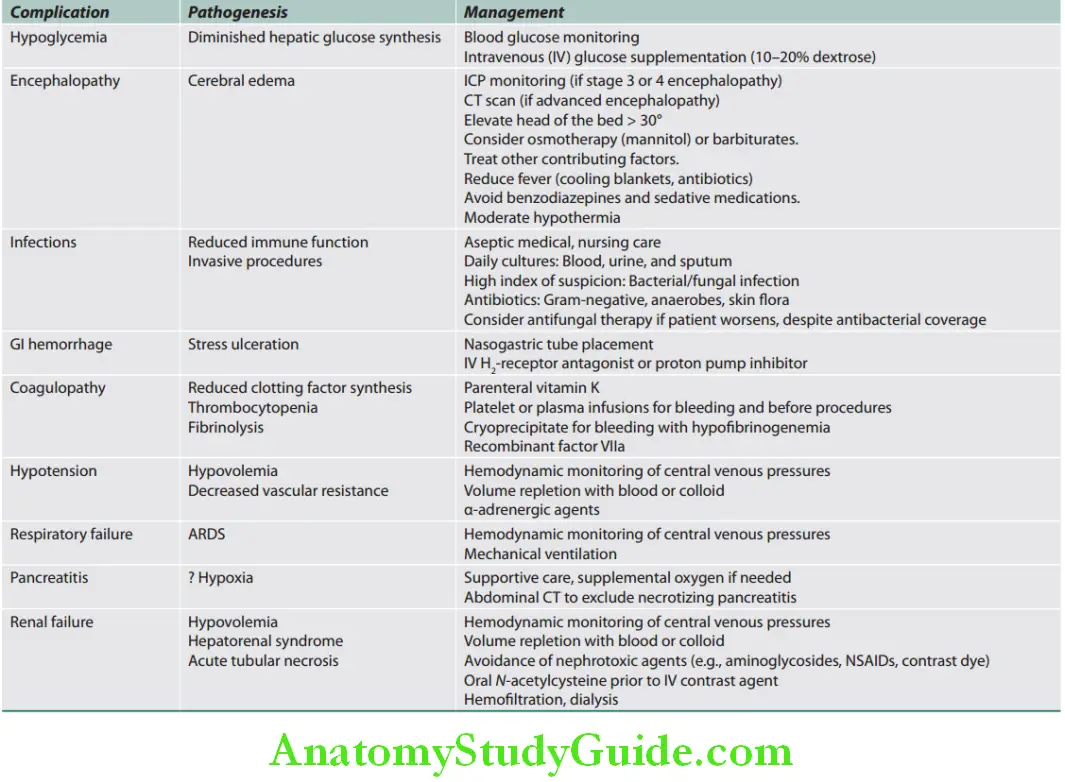
Prognosis: The mortality is ~80% without liver transplantation, and ~35% with transplantation.
Reye’s Syndrome:
- Reye/Reye’s syndrome is a rapidly progressive encephalopathy with hepatic dysfunction, which begins several days after apparent recovery from a viral illness, especially varicella or influenza A or B. History of aspirin intake may be present.
- Liver shows severe fatty change.
- Raised ammonia levels and liver enzymes
- Usually no jaundice
Fatty Liver:
Question 33. List causes of fatty liver.
Answer:
Fatty liver (steatosis) is an abnormal accumulation of triglycerides within the cytosol of the parenchymal cells.
Causes of fatty liver:
Various causes of fatty liver:
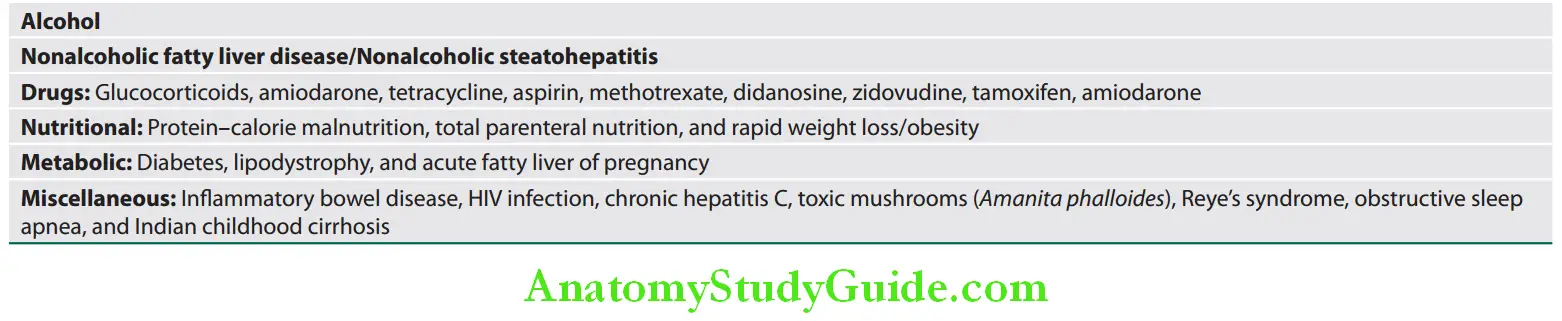
Nonalcoholic Fatty Liver Disease, Nonalcoholic Steatosis and Nonalcoholic Steatohepatitis):
Question 34. Write short note on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis.
Answer:
Nonalcoholic fatty liver disease is a disease of affluent societies. Its prevalence increases in proportion to the rise in obesity. Its progression accounts for the majority of cryptogenic cirrhosis.
It is increasingly recognized condition and is the most common cause of chronic liver disease after hepatitis B, hepatitis C, and alcohol.
Nonalcoholic Fatty Liver Disease Classifiation:
- Simple fatty liver disease with favorable prognosis
- Nonalcoholic steatohepatitis (NASH) associated with fibrosis and progression to cirrhosis and sometimes to HCC.
Risk Factors for NAFLD:
Increased prevalence in those with the metabolic syndrome.
- Obesity, hypertension, type 2 diabetes mellitus, hyperlipidemia, and insulin resistance.
- Rare causes: Tamoxifen, amiodarone and exposure to certain petrochemicals.
- Pathogenesis: NASH is induced by two consecutive steps: Excess fat accumulation and subsequent necroinflammation in the liver.
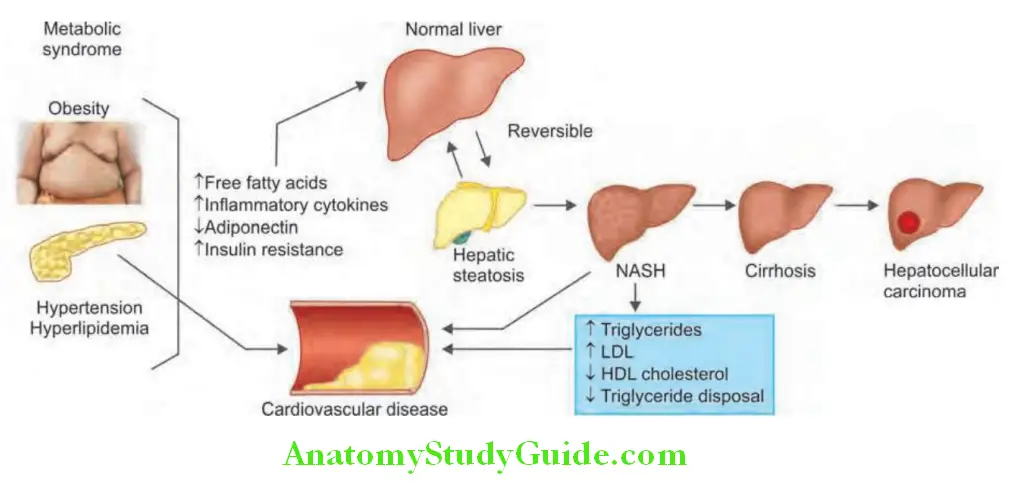
Nonalcoholic Fatty Liver Disease Clinical Features:
- Most are asymptomatic at the time of diagnosis and many patients are obese.
- Some have fatigue, malaise, and a sensation of fullness in the upper abdomen.
- Hepatomegaly is the only sign in most patients.
Diagnosis of NAFLD:
Patient with mild-to-moderately elevated serum transaminases, no history of alcohol abuse and a negative chronic liver disease screen.
Diagnosis of NAFLD Investigations:
- Mild elevation of serum aminotransferases is frequently the sole abnormality with AST: ALT < This ratio increases as fibrosis advances. It may be only isolated elevation of the GGT. ALP is elevated in about 30% of patients.
- The alcoholic liver disease to NAFLD index (ANI):
- ANI = -58.5 + 0.637 (MCV) + 91 (AST/ALT) 0.406 (BMI) + 6.35 for men
- An ANI greater than zero favors a diagnosis of alcoholic liver disease, whereas an ANI less than zero favors a diagnosis of NAFLD.
- Ferritin levels are increased in 20–50% of patients.
- Autoantibodies are found in about 25% patients with more advanced fibrosis.
- Ultrasound and CT features: Similar to those in alcoholic fatty liver
- Liver biopsy:
- Best diagnostic tool for confirmation and staging the disease.
- Microscopic changes are similar to those of alcohol-induced hepatic injury and range from simple fatty change to fat and inflammation (steatohepatitis) and fibrosis. NASH is characterized by fat, Mallory bodies, neutrophil infiltration, and pericellular fibrosis.
Diagnosis of NAFLD Management/Treatment:
- Weight loss, control of diabetes, and hyperlipidemia in the early stages
- Some drugs such as metformin, thiazolidinediones (e.g., pioglitazone), liraglutide, ursodeoxycholic acid (UDCA), vitamin E, orlistat, obeticholic acid, aramchol, betaine, losartan pentoxipylline and atorvastatin have shown some promise.
- Liver transplantation is done for end-stage cirrhosis.
- Unfortunately, it may recur in the graft.
- Regular follow-up, particularly for steatohepatitis
Alcoholic Liver Disease:
List the types of alcoholic liver diseases. Discuss the clinical features and management of alcoholic hepatitis.
Chronic and excessive consumption of alcohol can produce a wide spectrum of liver disease which can be divided mainly into four major lesions:
- Fatty liver
- Alcoholic hepatitis
- Alcoholic cirrhosis (refer later)
- HCC
Threshold for alcohol and risk of alcoholic liver disease: Generally, the effects of alcohol are worse in women compared to men and amount of alcohol with degree of risk in male are presented in Table.
Amount of alcohol consumption and its associated risk of alcoholic liver disease in male:
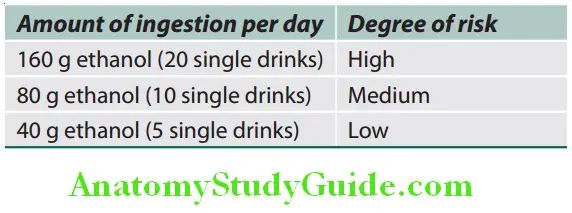
For women, the above figures should be reduced by 50%. Alcohol by volume (ABV) of various alcoholic beverages.
Alcohol percentage content:
- Vodka | ABV: 40–95%
- Gin | ABV: 36–50%
- Rum | ABV: 36–50%
- Whiskey | ABV: 36–50%
- Tequila | ABV: 50–51%
- Liqueurs | ABV: 15%
- Fortified Wine | ABV: 16–24%
- Unfortified Wine | ABV: 14–16%
- Beer | ABV: 4–8%
- Malt beverage | ABV: 5–15%
Alcoholic Fatty Liver (Alcoholic Steatosis):
Diagnosis of NAFLD Clinical Features:
- It is asymptomatic.
- Occasionally, it may present with discomfort in right upper quadrant, nausea, and jaundice.
- Most common feature is hepatomegaly with or without tenderness.
- Progression to cirrhosis is not common with its associated complications.
Diagnosis of NAFLD Investigations:
- Biochemical findings:
- Moderately raised ALT and AST with AST: ALT >
- γ-GT level is a sensitive test to determine whether the individual is taking alcohol.
- Ultrasound: Diffuse increase in echogenicity
- CT scan: Fatty infiltration produces a low-density liver.
- Liver biopsy: It shows accumulation of fat in perivenular hepatocytes and later in entire hepatic lobule.
Diagnosis of NAFLD Treatment: Complete cessation of alcohol consumption and nutritional support results in normalization of biochemical findings and histological changes.
Alcoholic Hepatitis:
Diagnosis of NAFLD Clinical Features:
- It may be asymptomatic or present with fever, rapid onset of jaundice, abdominal discomfort, and proximal muscle wasting.
- Portal hypertension, spider nevi, ascites, and bleeding due to esophageal varices can occur without cirrhosis.
- Hepatomegaly (tender) and splenomegaly.
Diagnosis of NAFLD Investigations:
- Biochemical findings:
- Serum aminotransferase (AST and ALT) raised to 2–7 times of normal (usually < 400 IU)
- AST: ALT ratio is >1 (generally >2)
- Raised bilirubin
- Mildly elevated serum ALP
- Decreased albumin
- Hematological findings: Prolonged prothrombin time and leukocytosis.
- Liver biopsy: Ballooning degeneration of hepatocytes with leukocyte infiltration. Mallory bodies are often seen. They are potentially reversible but many progress to cirrhosis.
Diagnosis of NAFLD Prognosis:
- Variable and despite abstinence, the liver disease progresses in many patients. Conversely, a few patients continue to drink heavily without developing cirrhosis.
- Mortality is high in patients with severe alcoholic hepatitis.
- Poor prognostic factors .
Poor prognostic factors of alcoholic hepatitis:
- Prothrombin time >5 seconds of control
- Anemia
- Albumin <5 g/dL
- Serum bilirubin >8 mg/dL
- Progressive encephalopathy
- Renal failure
- Presence of ascites
- Maddrey’s discriminant function >3
Maddrey’s discriminant function (DF) = (6 x [prothrombin time (sec) − control prothrombin time (sec)]) + (serum bilirubin) The ABIC (age, bilirubin, INR, and creatinine) is a modification of the MELD score. An ABIC score >9 is associated with approximately 80% mortality at 90 days.
Diagnosis of NAFLD Treatment:
- It is advised to stop alcohol consumption for life, because this is a precirrhotic condition.
- Severe hepatitis needs bed rest.
- Nutrition: Feeding via a fine-bore nasogastric tube or sometimes intravenously (>3,000 kcal/day; multivitamins mainly vitamins B and C).
- Treatment for encephalopathy and ascites
- Corticosteroids (prednisolone) may be tried in severe cases (discriminant function > 32) in the absence of any infection.
- Antibiotics (pentoxifylline) in severe cases (discriminant function >32) and antifungal prophylaxis.
Cirrhosis:
Question 35. LIst the causes of cirrhosis. Discuss the pathology, pathogenesis, classification, clinical features, investigations, complications and treatment/management of cirrhosis.
(or)
Describe Laennec’s cirrhosis and alcoholic cirrhosis.
Answer:
Cirrhosis Defiition:
Cirrhosis is an end-stage of any chronic liver disease. It is a diffuse process (entire liver is involved) characterized by fibrosis and conversion of normal architecture to structurally abnormal regenerating nodules of liver cells.
The three main morphologic characteristics of cirrhosis are:
- Fibrosis
- Regenerating Nodules
- Loss of architecture of the entire liver.
Cirrhosis Classifiation:
Morphological classification
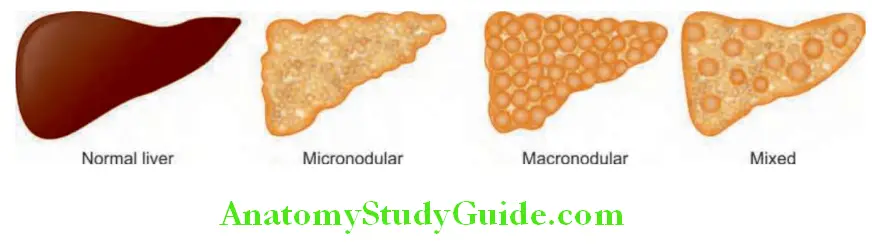
Morphological classification of cirrhosis:
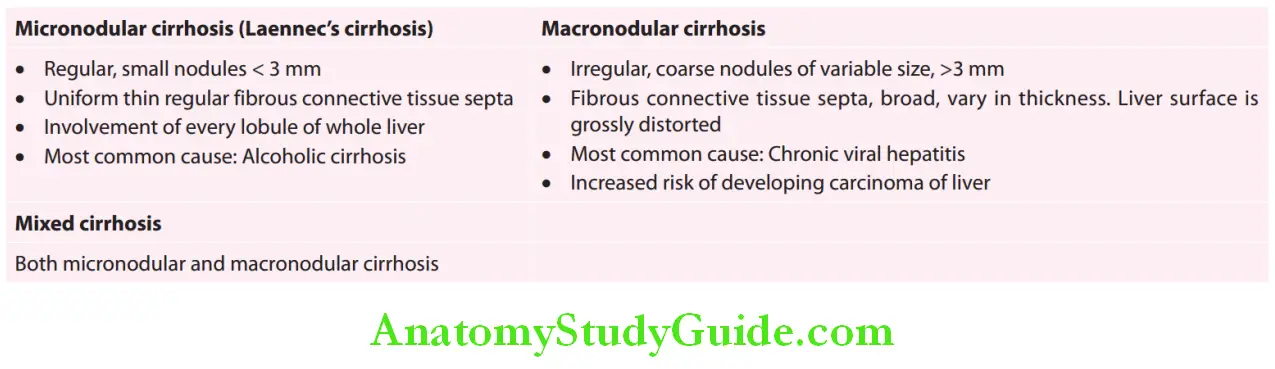
Cirrhosis Etiological Classifiation:
Main causes of cirrhosis:
- Alcohol (one of the most common causes)
- Chronic viral hepatitis (most common cause)
- Hepatitis B
- Hepatitis C
- Delta hepatitis (Hepatitis D) + Hepatitis B
- Nonalcoholic steatohepatitis (NASH) or nonalcoholic fatty liver disease (NAFLD) (earlier was considered as cryptogenic cirrhosis)
- Biliary cirrhosis
- Primary biliary cholangitis
- Secondary biliary cirrhosis
- Primary sclerosing cholangitis
- Autoimmune cholangiopathy, IgG4 cholangiopathy
- Autoimmune hepatitis
- Budd–Chiari syndrome
- Intrahepatic or extrahepatic biliary obstruction: Recurrent biliary obstruction (e.g., gallstones)
- Inherited metabolic liver disease
- Hemochromatosis
- Wilson’s disease
- α1 antitrypsin deficiency
- Cystic fibrosis
- Glycogen storage disease
- Drug-induced cirrhosis: For example, methotrexate, methyldopa, isoniazid, phenylbutazone, sulfonamides
- Others: Indian childhood cirrhosis, cardiac cirrhosis, chronic venous outflow obstruction, celiac disease Hereditary hemo-telangiectasia, infection [e.g., brucellosis, syphilis, echinococcosis, porphyria, idiopathic adulthood ductopenia (Caroli disease)]
Pathology and Pathogenesis of Cirrhosis:
- Chronic injury to the liver results in inflammation and widespread necrosis of liver cells and, eventually, fibrosis. Fibrosis is due to activation of the stellate cells (in the space of Disse) by many cytokines and their receptors, reactive oxygen intermediates, and other paracrine and autocrine signals. TGF-β is the most potent fibrogenic mediator.
- Cirrhotic changes affect the whole liver, but not necessarily every lobule.
- Extensive fibrosis that distorts and results in loss of liver architecture.
- Regenerating nodules are produced due to hyperplasia of the remaining surviving liver cells.
- Destruction and distortion of hepatic vasculature by fibrosis lead to obstruction of blood flow. Vascular reorganization leads to portal hypertension and its sequelae (gastroesophageal varices and splenomegaly).
- Ascites and hepatic encephalopathydevelop due to hepatocellular insufficiency and portal hypertension.
- Hepatocellular damage produces jaundice, edema, coagulopathy, and a various metabolic abnormalities.
Alcoholic Cirrhosis:
- Safe limits of alcohol are 200 g (20–40 g/day) in males and 140 g (16–30 g/day) in females of alcohol per week. Intake of 180 g of alcohol/day for 25 years increases the risk of cirrhosis by 25 times.
- Development of cirrhosis is six-fold when alcohol consumption is double the safety limit.
- Hepatitis C infection is an important contributory factor for progression to cirrhosis.
- Pathogenesis of alcoholic cirrhosis.

Clinical Features:
Question 36. Write the clinical features and treatment of alcoholic cirrhosis.
Answer:
Alcoholic cirrhosis Symptoms:
- Highly variable and in some patients it may be completely asymptomatic and is incidentally diagnosed at ultrasound or at surgery.
- Nonspecific symptoms: Weakness, fatigue, muscle cramps, weight loss, anorexia, nausea, vomiting, and upper abdominal discomfort.
- Symptoms of hepatic insufficiency
- Symptoms of portal hypertension and its sequelae
- Symptoms due to endocrine changes:
- Loss of libido, hair loss
- Females: Irregular menses, amenorrhea, and atrophy of breast
- Males: Gynecomastia, testicular atrophy, and impotence.
- Hemorrhagic tendencies: Due to decreased production of coagulation factors by the liver and thrombocytopenia resulting from hypersplenism. These include easy bruising, purpura, epistaxis, menorrhagia, and GI bleeding.
Alcoholic cirrhosis Signs:
Summary of signs of cirrhosis of liver:

Jaundice:
- Jaundice is not a common feature of cirrhosis; it is more common with acute diseases.
- Mechanisms of jaundice in cirrhosis:
- Failure to excrete bilirubin (mainly)
- Intrahepatic cholestasis (superadded hepatitis/tumor)
- Hemolysis due to hypersplenism (not a major contributor)
Hepatomegaly:
- Early stages: Liver is enlarged, firm to hard, irregular, and nontender. Hepatomegaly is not common in cirrhosis but common when the cirrhosis is due to alcoholic liver disease, NASH, and hemochromatosis. Hepatomegaly may indicate transformation into HCC.
- Late stages: Liver decreases in size and nonpalpable due to progressive destruction of liver cells and accompanying fibrosis.
Ascites:
Ascites due to liver failure and portal hypertension signify advanced disease.
Circulatory changes:
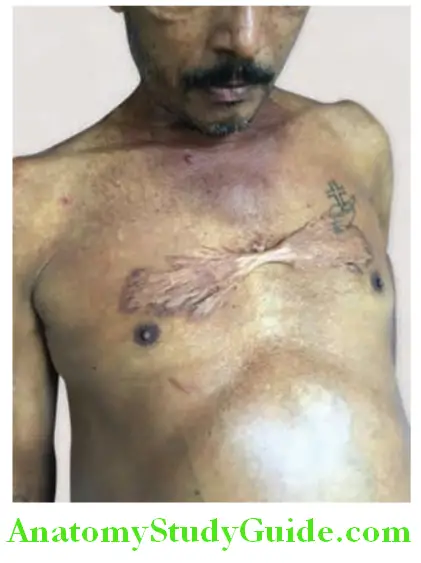
1. Spider nevi (Spider telangiectasia, vascular spiders, spider angiomas, arterial spiders).
- Appearance: Consists of a central arteriole from which numerous small vessels radiate peripherally-resembling spider’s legs. Whole spider disappears when central arteriole is compressed with a pinhead. When compression is released filling occurs from center to periphery.
- Cause: Due to arteriolar changes induced by hyperestrogenism.
- Sites affected: Usually found only in the necklace area, i.e., above the nipples, territory drained by the superior vena cava such as head and neck, upper limbs, front and back of upper chest. Most probable cause of location in the superior vena cava drainage area in that maximum blood in the portal circulation that cannot go through the liver due to fibrosis reach the systemic circulation through the SVC.
- Size: Varies from pinhead to 0.5 mm in diameter.
- Significance: They are a strong indicator of liver disease but can be found in other conditions.
- Florid spider telangiectasia, gynecomastia, and parotid enlargement are most common in alcoholic hepatitis.
- Florid spiders and new-onset clubbing in a patient with cirrhosis indicate hepatopulmonary syndrome.
- Differential diagnosis for spider nevi includes venous star, Campbell de Morgan spots, petechiae, and hereditary hemorrhagic telangiectasias.
2. Palmar erythema (liver palm):
- Can be seen early but is of limited diagnostic value, as it occur in many conditions associated with a hyperdynamic circulation (e.g., normal pregnancy).
- Cause: Develops due to increased peripheral blood flow. In cirrhosis, circulatory changes results in increased peripheral blood flow and decreased visceral blood flow (especially to the kidneys).
- Sites involved: Prominent in the thenar and hypothenar eminences of palm. May be seen on the sole.
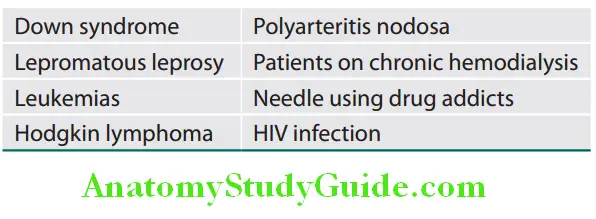
Conditions associated with spider nevi:
Endocrine changes:
- Diminished body hair and loss of hair:
- Seen mainly in males with loss of male hair distribution
- Alopecia affects usually the face, axilla, and chest and is due to hyperestrogenism
- Causes of hyperestrogenism: Due to increased peripheral formation of estrogen resulting from diminished hepatic clearance of the precursor, androstenedione.
- Effects of hyperestrogenism: Alopecia, gynecomastia, and testicular atrophy.
- Hyperglycemia: 80% of cirrhotics have impaired glucose tolerance, 20% develop diabetes.
In Males:
Question 37. Write short note on gynecomastia and enumerate its causes.
Answer:
- Gynecomastia:
- Found in males (atrophy of breasts in females).
- Cause: Due to increased estradiol/free testosterone ratio
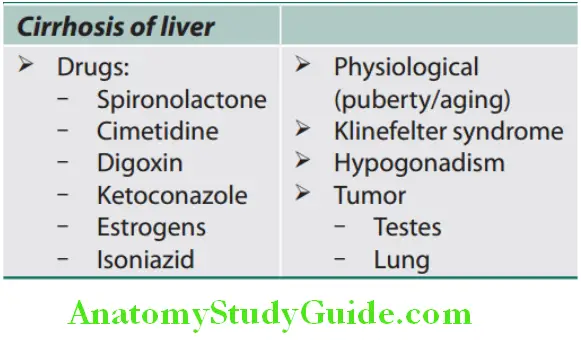

-
- Appear as palpable nodule (4 cm, subareolar) Microscopy: Proliferation of glandular tissue of breast. Pseudogynecomastia is accumulation of subareolar fat tissue without palpable nodule.
- Testicular atrophy: Due to hyperestrogenic state, it is characterized by a small size compared with Prader orchidometer —soft testes with loss of testicular sensation (sickening sensation in epigastrium on squeezing the testes). The dimensions of the average adult testicle is 5 × 5 × 5 cm and the volume is 15–25 mL.
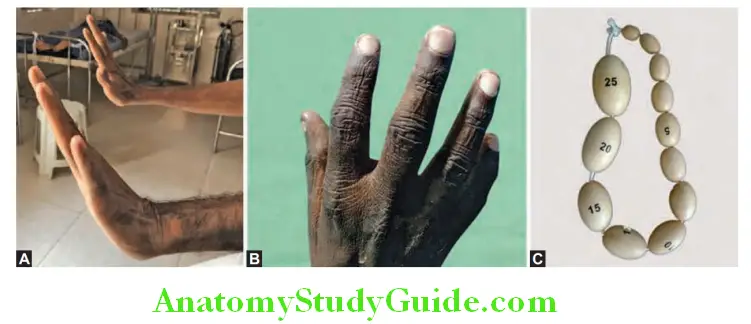
In Females:
- Irregular menses, amenorrhea, and atrophy of breast.
Flapping tremors: Observed in hepatic precoma (refer to hepatic encephalopathy on pages 832–7).
In Females Other features:
- Generalized skin hyperpigmentation: Due to increased melanin deposition
- Dupuytren’s contracture—sign of alcoholism
- Cause: Fibrosis of palmar aponeurosis is probably caused by local microvessel ischemia. Platelet and fibroblastderived growth factors promote fibrosis.
- Sites involved: Flexion contracture of the fingers (especially ring and little fingers)
- Other causes of Dupuytren’s contracture: Diabetes mellitus, rheumatoid arthritis, and manual labor (workers exposed to repetitive handling tasks or vibration).
- Clubbing and central cyanosis: Due to development of pulmonary arteriovenous shunts that lead to hypoxemia
- Nail changes:
- White (Terry’s) chalky and brittle nails
- Muehrcke’s nails: Characterized by transverse white lines that disappear on applying pressure and these lines do not move with growth of nail.
- Clubbing is present in PBC or hepatoma
- Parotid and lacrimal gland enlargement: Observed commonly in alcoholic cirrhosis due to associated autonomic dysfunction.
- Anemia: Due to various causes
Causes of anemia in cirrhosis:
- Acute and chronic blood loss from varices
- Nutritional deficiency of vitamin B12 and folate
- Hypersplenism
- Bone marrow suppression by alcohol
- Hemolysis.
- Zeive’s syndrome: Alcohol-induced hemolytic anemia with hypercholesterolemia
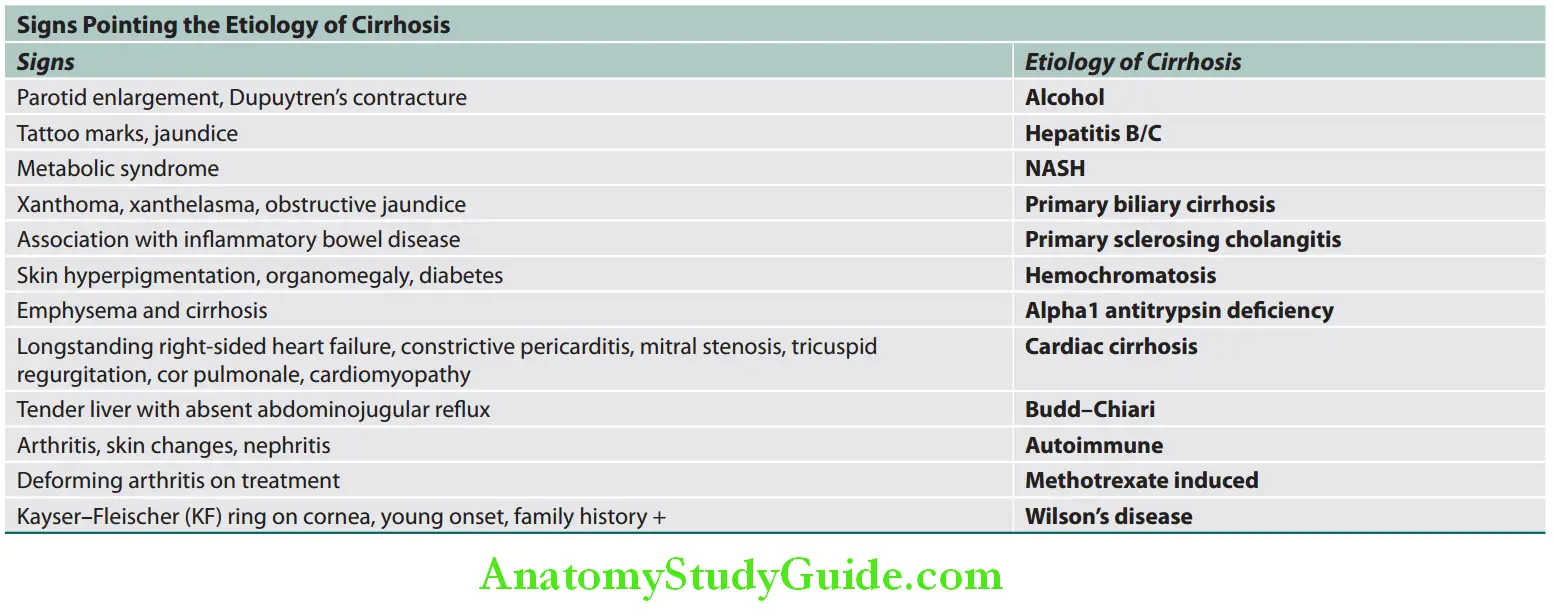
Symptoms and Signs due to Complications:
Question 38. Write short essay on complications/life-threatening complications of cirrhosis of liver.
Answer:
Three major complications of cirrhosis are:
- Portal hypertension: Splenomegaly and collateral vessel formation are features of portal hypertension develops in advanced disease.
- Hepatic encephalopathy
- Renal failure.
Complications of cirrhosis:

Extrahepatic manifestations:
- Pleural effusion (hepatic hydrothorax):
- It is transudate. Often associated with ascites. Most often seen on right side.
- Mechanisms:
- Hypoalbuminemia causes decreased colloid osmotic pressure.
- Leakage of ascitic fluid may occur through diaphragmatic defects.
- Transdiaphragmatic migration of fluid via lymphatic channels
Extrahepatic manifestations Treatment: It includes control of ascites, transjugular intrahepatic portosystemic shunt, video-assisted thoracoscopy with pleurodesis, video-assisted thoracoscopy with repair of defects in the diaphragm and liver transplantation.
2. Hepatopulmonary syndrome:
- Definition: Hypoxemia occurring in patients with advanced liver disease
- Cause: Hypoxemia is due to intrapulmonary vascular vasodilation without any evidence of primary pulmonary disease.
- Patients show features of cirrhosis with spider nevi, clubbing, and cyanosis.
- Symptoms: Most patients have no respiratory symptoms but with more severe disease complain of insidious onset of breathlessness on standing (orthodeoxia–platypnea).
- Investigations:
- Chest X-ray: May show a bibasilar interstitial pattern that reflects the predominantly basal vascular dilatations.
- Transthoracic ECHO: Shows intrapulmonary shunting.
- Arterial blood gases: Confirm the arterial oxygen desaturation. Diagnosis by contrast-enhanced (microbubble) echocardiography, perfusion lung scan, and pulmonary angiography.
Hepatopulmonary syndrome Treatment: Oxygen inhalation and coil embolization (in localized shunts).
Liver transplantation.
3. Portopulmonary hypertension: It is characterized by a raised mean pulmonary artery pressure, increased pulmonary vascular resistance and normal wedge pressure developing in portal hypertension. Dyspnea on exertion is the most common symptom, and can lead to right heart failure.
4. Cirrhotic cardiomyopathy: Characterized by systolic and diastolic dysfunction, electrophysiological changes, and gross and microscopic structural changes.
5. Hepatic osteodystrophy: Osteoporosis and osteomalacia
Noninvasive direct and indirect markers of hepatic firosis:

Prognostic Classifiations:
Question 39. Write short note on Child–Pugh score or Child–Turcotte–Pugh score.
Answer:
The Child–Pugh (CP) scoring classification was originally used to risk-stratify patients undergoing shunt surgery. Modifications of Child’s–Pugh grading (A, B, and C)/Child–Turcotte–Pugh score is shown in and is useful to grade the severity of liver disease and prognosticate patients with established cirrhosis. Other scoring system used is The Model for End-stage
Liver Disease (MELD) which bilirubin, creatinine, and INR for prothrombin time to predict 3-month survival. MELD = 8 × loge(serum bilirubin [mg/dL]) + 12 × loge(INR) + 9.6 × loge(serum creatinine [mg/dL]) + 6.4
Modifid Child’s–Pugh classifiation or Child–Turcotte–Pugh (CTP) score:
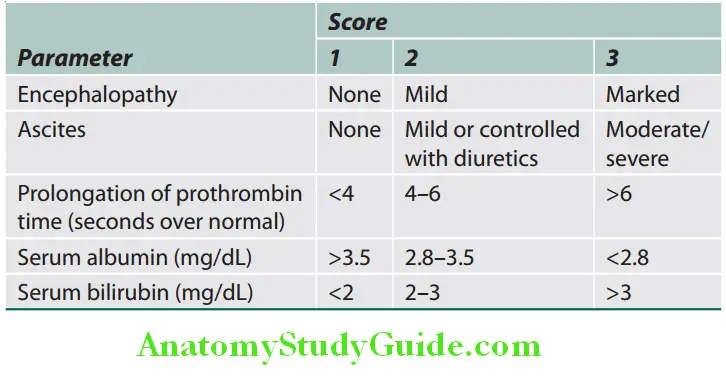
Characteristics of end-stage of cirrhosis:
- Jaundice
- Progressive, refractory ascites
- Worsening of signs of portal hypertension
- Progressive renal dysfunction hepatic encephalopathy
Poor Prognostic Factors in Cirrhosis:

Prognostic Classifiations Investigations:
Investigations are helpful for assessing the severity and type of liver disease.
Liver Function Tests:
- Hyperbilirubinemia: Due to rise in both conjugated and unconjugated bilirubin. Not very common with cirrhosis. It could suggest superadded hepatitis, hepatocellular carcinoma, congestive or obstructive etiology.
- Serum proteins: Show reversal of A:G ratio.
- Serum albumin is decreased (hypoalbuminemia) and is due to reduced synthesis by liver.
- Serum globulin is increased (hyperglobulinemia) due to stimulation of reticuloendothelial system.
- Serum transaminases:
- AST (SGOT) is raised.
- ALT (SGPT) is raised and usually less than 300 units/dL.
- AST:ALT ratio
- More than 2 in alcoholic cirrhosis.
- Less than 2 in cirrhosis complicating viral hepatitis.
- ALP: It may be slightly elevated.
- Prothrombin time: Prolonged due to reduced synthesis of clotting (especially vitamin K-dependent) factors.
Hematological Tests:
Peripheral blood picture:
- Anemia and acanthocytosis (spur-like projections on RBC).
- Leukopenia and thrombocytopenia (due to hypersplenism and bone marrow suppression by alcohol).
Serological markers: For hepatitis B and C.
Other Biochemical Markers:
- Serum electrolytes:
- Low sodium (hyponatremia) indicates severe liver disease and is due to either defect in free water clearance or to excess diuretic therapy.
- Hypokalemia, hypomagnesemia, and hypophosphatemia
- Blood ammonia estimation: It is a reliable investigation when hepatic encephalopathy is suspected. Raised blood ammonia is due to:
- Decreased clearance by liver
- Shunting of portal venous blood to systemic circulation
- Respiratory alkalosis: It may develop due to central hyperventilation.
- Glucose intolerance.
Imaging:
- Ultrasound examination can demonstrate features listed.
- CT scan: To detect hepatosplenomegaly and dilated collaterals. Arterial phase-contrast-enhanced scans to detect HCC. Other noninvasive markers to detect hepatic fibrosis are Hepascore, FIB-4 index, Fibroindex, AST to platelet ratio, etc.
- Endoscopy for detecting and treating varices and portal hypertensive gastropathy. Barium swallow may also be useful for demonstration of varices.
- Hepatic elastography
Ultrasound examination in cirrhosis:
- Changes in size and shape of the liver
- Fatty change and fibrosis produce a diffuse increased echogenicity
- Nodularity of the liver surface
- Distortion of the arterial vascular architecture
- Patency and size of the portal and hepatic veins and their diameters
- Detect hepatocellular carcinoma
- Enlargement of spleen
- Ascites
Question 40. Write short note on hepatic elastography.
Answer:
- Hepatic fibrosis represents an early stage of chronic liver disease and cirrhosis.
- Conventional liver tests and imaging studies are not sensitive for detecting hepatic fibrosis.
- Hepatic elastography is a noninvasive method for measurement of hepatic fibrosis.
- Methods used are ultrasound and MRI [acoustic radiation force impulse imaging (ARFI), real-time shear wave elastography (SWE)].
- Methods for performing SWE, including transient elastography, point-SWE, and two-dimensional (2D)-SWE.
- Ultrasound-based elastography is primarily used to assess hepatic fibrosis, it can also be used to predict complications in patients with cirrhosis-like development of large varices and HCC.
Liver Biopsy:
- Usefulness of liver biopsy in cirrhosis
- To confirm the diagnosis of cirrhosis
- Assesses the severity and type of liver disease
- Special stains: They may be necessary for iron and copper
- Immunocytochemical stains: They can identify viruses.
Special investigations depending on the etiology:
- Chemical measurement of iron (serum transferrin saturation level and serum ferritin) and copper (ceruloplasmin) are required to confirm diagnosis of iron overload or Wilson’s disease.
- Others: Serum α-fetoprotein, α1-antitrypsin, antinuclear antibodies and anti-smooth muscle antibodies, etc. depending on the etiology.
Ascitic Fluid Examination (Discussed Later):
Investigations for the etiology of cirrhosis: Even in a patient of cirrhosis with chronic consumption of alcohol, rule out other causes (viral serology, etc.) as only 15–18% of alcoholics develop cirrhosis.
Hepatic elastography Management (Treatment):
- There is no treatment available to arrest or reverse the cirrhotic changes in liver. Liver transplantation is the specific treatment.
- Progression may be halted by correcting the underlying cause, removal of causative agents such as drugs and alcohol.
- Diet:
- High-protein diet: Minimum 1 g/kg/day; 2,000–3,000 kcal/day
- Diets with branched-chain amino acids in patients predisposed to hepatic encephalopathy.
- Multivitamin supplements.
- Reduce salt intake.
- Avoidance of aspirin, NSAIDs, and other hepatotoxic drugs.
- Management of the complications: Specific treatment of complications, e.g., variceal bleeding, hepatic encephalopathy, and ascites (discussed separately).
- Follow-up: With 6-monthly ultrasound and serum α-fetoprotein measurements for early detection of development of a HCC.
Portal Hypertension:
Portal vein is formed by the union of the superior mesenteric and splenic veins. Normally, the pressure within portal vein is 5–8 mm Hg (or 10–15 cm saline).
Hepatic elastography Definition:
- Portal hypertension is defined as prolonged elevation of portal venous pressure (>30 cm saline).
- It is better defined as elevation of hepatic venous pressure gradient (HVPG-difference in pressure between portal vein and hepatic vein) more than 7 mm Hg. HVPG more than 10 mm Hg defines significant portal hypertension.
Classification of Portal Hypertension:
Question 41. Define and classify portal hypertension.
Answer:
Portal hypertension can be classified according to the site of obstruction into prehepatic, intrahepatic, and posthepatic.
- Prehepatic causes: Obstruction/blockage of the portal vein before it ramifies within the liver, e.g., portal vein thrombosis, splenic vein thrombosis, and massive splenomegaly (Banti’s syndrome).
- Intrahepatic causes: Due to distortion of the liver architecture and may be further divided into:
- Presinusoidal (e.g., in schistosomiasis)
- Sinusoidal (e.g., in cirrhosis)
- Postsinusoidal (e.g., hepatic sinusoidal obstruction—venoocclusive syndrome).
- Posthepatic causes: Due to venous blockage outside the liver and are rare, e.g., severe right-sided heart failure, Budd–Chiari syndrome, constrictive pericarditis, and hepatic vein outflow obstruction.

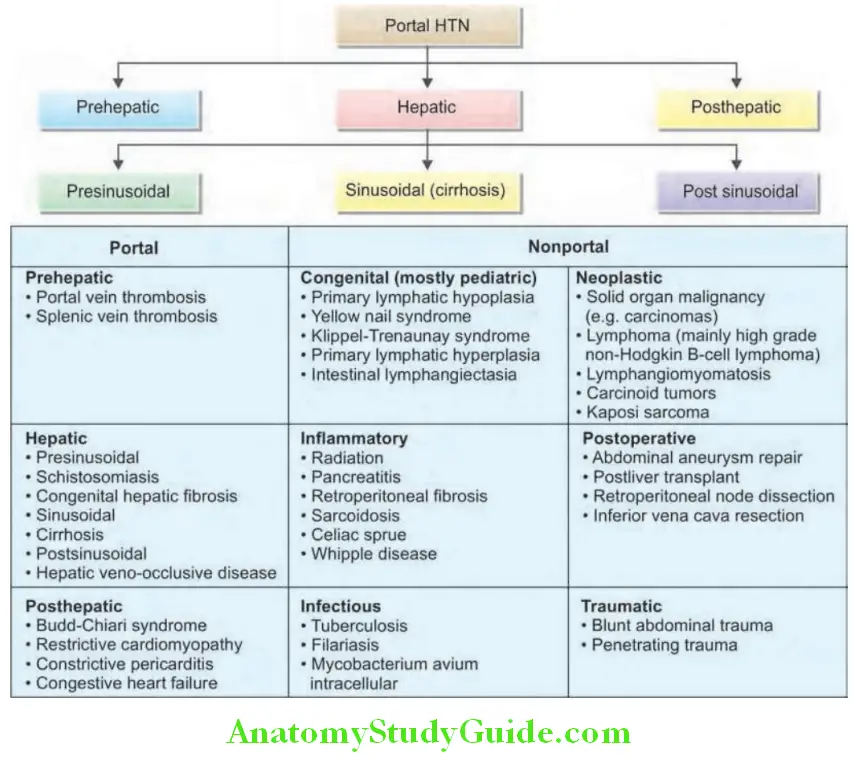
Pathogenesis of Portal Hypertension:
Question 42. Discuss the etiology, pathogenesis, clinical features, investigations, complications and management of portal hypertension.
Answer:
Portal hypertension in cirrhosis results from:
- Increased intrahepatic resistance to blood flow through the liver and leads to:
- As portal pressure rises above 10–12 mm Hg, the portal venous system dilates, reduces the portal blood to the liver and collaterals occur within the systemic venous system. Development of collateral vessels allowing portal blood to bypass the liver and enter systemic circulation.
- Initially, collateral vessel formation diverts most of the portal blood and later almost all of the entire portal blood directly to the systemic circulation, bypassing the liver.
- Increase in portal venous inflow (flow) resulting from the hyperdynamic circulation.

Clinical Features:
Question 43. Write short note on clinical features and diagnosis of portal hypertension.
Answer:
- Patients with portal hypertension are often asymptomatic.
- History, e.g., alcoholism, past history of hepatitis. Triad of portal hypertension consists of ascites, splenomegaly, and dilated tortuous abdominal wall veins.
- Bleeding from esophageal varices or portal hypertensive gastropathy causes hematemesis and melena.
- Splenomegaly: It may be only clinical evidence and single most important diagnostic feature of portal hypertension.
- A diagnosis of portal hypertension should not be made if there is no documentation of splenomegaly either clinically, radiographically or ultrasonography.
- Usually spleen is palpable less than 5 cm below costal margin. However, marked splenomegaly can be found in younger patients, those with macronodular cirrhosis and in extrahepatic portal hypertension.
- Hypersplenism manifests as thrombocytopenia and leukopenia. Splenomegaly may disappear after massive GI bleed or shunt surgery.
- Features due to liver cell failure/hepatic encephalopathy.
- Fetor hepaticus: It is a musty odor of breath due to shunting of blood from portal system to systemic circulation that allows mercaptans to pass directly to the lungs, bypassing the liver.
- Caput medusae, suggestive of an intrahepatic cause of portal hypertension, are present around the umbilicus; the flow of blood is away from the umbilicus.
- In Budd–Chiari syndrome, by contrast, veins are dilated in the flanks and back, and blood flows in a cephalic direction.

- A bruit may be heard in the left or the right upper quadrant in a patient with a splanchnic arteriovenous fistula.
- Cruveilhier–Baumgarten syndrome: A venous hum may be heard in the epigastrium of a patient with portal hypertension and represents collateral flow in the patent umbilical vein in the falciform ligament. A patent umbilical vein excludes an extrahepatic cause of portal hypertension because the umbilical vein arises from the intrahepatic portion of the left portal vein.
- Rectal varices may be confused with internal hemorrhoids because of their location. However, hemorrhoids result from a displacement of the anal cushions and hyperperfusion of the arteriovenous plexus vascular cushions without direct communication with any of the major branches of the portal venous system.
- Liver: It may be enlarged or shrunken.
- Small, contracted, fibrotic liver is found when the portal venous pressure is very high.
- Soft liver usually suggestive of extrahepatic portal vein obstruction.
- Firm liver suggestive of cirrhosis and hence intrahepatic portal hypertension
- Ascites: It develops partly due to portal hypertension but mainly due to liver cell failure.
Portal hypertension Investigations:
The diagnosis of portal hypertension can be made on clinical grounds.
- Barium swallow: It shows varices as filling defects in the lower-third of esophagus (“bag of worms appearance”).
- Upper GI scopy
- Most reliable method of investigation.
- Esophageal varices appear as blue rounded projections (red spots and red stripes) under submucosa.
- “Cherry red spots” indicate impending rupture of varices.
- Ultrasonography.
- Portal venography (splenoportal venogram) rarely done nowadays.
- Measurement of portal venous pressure: By either wedged hepatic venous pressure (WHVP) or transhepatic venous pressure. It is useful for
- Confirmation Of Portal Hypertension
- Differentiating sinusoidal from presinusoidal portal hypertension.
- Proctoscopy and barium enema: Useful for demonstrating varices in the rectum and colon
- Liver function tests: To confirm the liver diseases.
Complications of Portal Hypertension:
- Variceal bleeding: Mainly esophageal and gastric
- Hepatic encephalopathy
- Ascites, spontaneous bacterial peritonitis (SBP)
- Hepatorenal, hepatopulmonary syndrome
- Congestive gastropathy
- Hypersplenism
- Iron-deficiency anemia
Question 44. Name the drugs that are used in the reduction of portal venous pressure.
Answer:
Treatment of Portal Hypertension:
- Treatment of underlying disease
- Drugs used in the treatment of portal hypertension.
- Nonselective β-blockers (propranolol, nadolol) produce vasodilatation of both splanchnic arterial bed and portal venous system. Also reduce recurrence of variceal bleed. The usual starting dose of long-acting propranolol is 40 mg once daily and that of nadolol is 20 mg once a daily. Carvedilol in addition to reducing portal venous inflow through nonselective beta blockade, the anti-alpha 1 adrenergic activity leads to reduced hepatic vascular tone and hepatic resistance.
- Nitrates (nitroglycerine and isosorbide dinitrate) along with β-blockers reduce the risk of variceal bleed and are used for primary prophylaxis of variceal bleed.
Drugs used in the treatment of portal hypertension:
- Drugs that decrease portal blood flow:
- Nonselective β-adrenergic blocking agents (propranolol, nadolol, carvedilol)
- Somatostatin and its analogs
- Vasopressin
- Drugs that decrease intrahepatic resistance
- α1-adrenergic blocking agents (e.g., prazosin)
- Angiotensin receptor blocking agents
- Nitrates
Esophageal Varices:
Question 45. Discuss the diagnosis and management of esophageal variceal bleeding.
Answer:
- About 90% of patients with cirrhosis will develop gastroesophageal varices, but only one-third of these will bleed from them.
- The most common site of bleeding is esophageal varices within 3–5 cm of the esophagogastric junction.
- Factors that predispose to rupture of varices are listed.
Factors predisposing varices rupture:
- Large varices
- “Red sign” on varices (diagnosed at endoscopy) suggests imminent rupture.
- Associated with severe liver disease
- High portal venous pressure
- Salicylates and other nonsteroidal anti-inflammatory drugs
Esophageal Varices Clinical Features:
Painless, mild-to-massive hematemesis, with or without associated melena
Esophageal Varices Signs:
- Depending on the amount of blood loss, it may vary from mild postural tachycardia to shock.
- Features of liver cell failure, ascites, and portal hypertension.
Esophageal Varices Diagnosis:
- Fiberoptic endoscopy: Performed within 8 hours of bleeding reveals the bleeding site and the presence of varices. This helps in excluding the other causes of bleeding. The descriptors are presented.
- Ultrasonography: Useful to confirm the patency of portal vein.

Descriptors of esophageal varices:
- Red color signs (ominous signs) include:
- Red “wale” markings, which are longitudinal whip-like marks on the varix
- Cherry-red spots, which usually are 2–3 mm or less in diameter
- Hematocystic spots are blood-filled blisters 4 mm in diameter
- Color of the varix can be white or blue
- Form (size) of the varix at endoscopy:
- Grade 1: Esophageal varices may be small and straight
- Grade 2: Tortuous and occupying less than one-third of the esophageal lumen
- Grade 3: Large and occupying more than one-third of the esophageal lumen
Question 46. Describe the management of acute variceal bleeding. Describe the medical management of bleeding of esophageal varices.
Answer:
Esophageal varices Management:
Management goal:
Management goal in esophageal varices:
- Management of the active bleeding episode
- Prevention of rebleeding
- Prophylactic measures to prevent the first hemorrhage
Management of acute variceal bleeding.
General measures—resuscitation:
- Immediate hospitalization and nursing: Patients require intensive-care nursing. Nil by mouth until bleeding stops.
- Assess the general condition of the patient pulse and blood pressure and maintain fluid (intake and output) and electrolyte balance.
- Obtain blood for grouping and crossmatching, hemoglobin, prothrombin time, blood urea, electrolytes, creatinine, LFTs, and blood cultures.
- Grade cirrhosis according to Child-Pugh score.
- Immediately restore blood volume with blood transfusion and avoid saline infusions as far as possible. Prompt correction of hypovolemia is needed.
- Correction of coagulation factor deficiency by fresh blood or fresh frozen plasma.
- Platelet transfusions to raise platelet count above 50,000/mm
- Vitamin K is administered intramuscularly or intravenously.
- To prevent stress ulcers, give H 2 receptor antagonists (e.g., cimetidine, ranitidine or famotidine) or proton-pump inhibitors (e.g., pantoprazole or omeprazole).
- Prophylactic antibiotics: Reduce infection and mortality and prevent spontaneous bacterial peritonitis (SBP). Usually oral and intravenous quinolones are used (e.g., ciprofloxacin 500 mg twice daily
- Measures to prevent hepatic encephalopathy: Portosystemic encephalopathy (PSE) may be precipitated when the amount of bleeding is large because blood contains protein.
- Treatment of ascites by ascitic tap (paracentesis) or by administration of spironolactone or amiloride.
- Monitor for alcohol withdrawal (in case of alcoholic cirrhosis) and give thiamine.
Urgent endoscopy:
- Urgent endoscopy is done to confirm the diagnosis of gastroesophageal varices. Varices may or may not be present and it helps to exclude bleeding from other sites (e.g., gastric ulceration).
- Portal hypertensive (or congestive) gastropathy is defined as chronic gastric congestion, punctate erythema, and gastric erosions. It can also cause bleeding.
- To reduce esophageal ulceration following endoscopic therapy, sucralfate is given in the dose of 1 g four times daily.
Local measures:
Injection sclerotherapy or variceal banding Acute variceal bleeding may be treated by endoscopic procedure with
- Sclerotherapy
- Endoscopic Variceal Banding [Endoscopic Variceal Band Ligation (EVBL)].
- They arrest bleeding in 80% of cases and reduce early rebleeding.
- Sclerotherapy: Injection of sclerosing agent into the varices may arrest bleeding by producing thrombosis of vessel. Commonly used sclerosants are listed. and complications of sclerotherapy are mentioned.
- Banding: Varices are banded by mounting a band on the tip of the endoscope, sucking the varix just into the end of the scope and dislodging the band over the varix.
- Cyanoacrylate injection is used in patients with large gastric varices.
Vasoconstrictor therapy:
- Uses: This therapy is mainly used for
- Emergency Control Of Bleeding While Waiting For Endoscopy
- In combination with endoscopic techniques.
Aim: To reduce the portal inflow of blood and portal pressure constricting the splanchnic arterioles.
Drugs used:
- Terlipressin:
- Action: Terlipressin produces vasoconstriction by releasing vasopressin from it.
- Dose: 2 mg IV 6-hourly till bleeding stops, and then reduced to 1 mg 4-hourly after 48 hours if a prolonged dosage regimen is used.
- Contraindication: Patients with ischemic heart disease.
- Side effects: Abdominal colic, evacuation of bowels, and facial pallor due to the generalized vasoconstriction.
- Somatostatin and octreotide:
- Somatostatin and its synthetic analog octreotide stops bleeding from varices in more than 80% patients.
- Its effects are equivalent to vasopressin and endoscopic therapy.
- Side-effects: Very few.
- Dose:
- Somatostatin: Infusion of 250–500 μg/h followed by 250 μg/h for 2–5 days.
- Octreotide: 50 μg as bolus followed by 50 μg/h for 2–5 days.
- Indication: When there are contraindications to terlipressin.
- Vasopressin [0.1–0.5 units/min for 4–12 hours (up to 48 hours)]: It was used in the past but is not commonly used now.
Commonly used sclerosants in esophageal varices:
- Ethanolamine oleate
- Sodium morrhuate
- Absolute alcohol
- Sodium tetradecylsulfonate
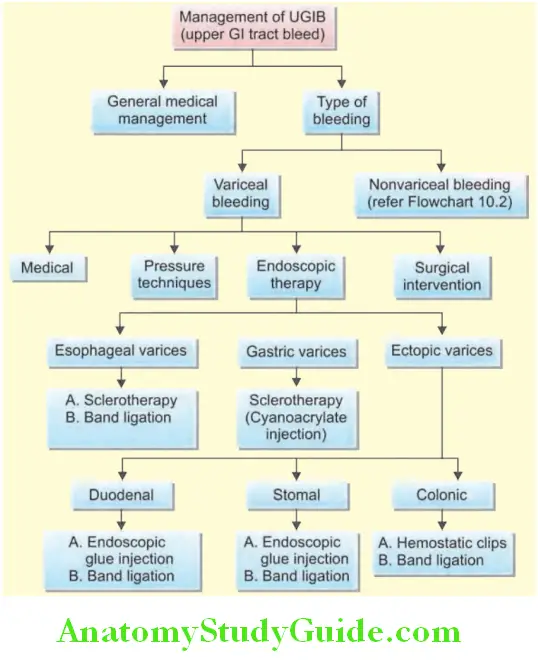
Balloon Tamponade:
- Indications: To control variceal bleeding
- If endoscopic therapy or vasoconstrictor therapy fails or contraindicated
- If there is exsanguinating hemorrhage
- Instrument:
- Sengstaken–Blakemore tube with four lumens
- Minnesota tube with four lumens. These tubes have two balloons, namely esophageal and gastric balloons.
- Use: It is very useful in the initial few hours of bleeding in about 90% of cases.
- Complications:
- Aspiration pneumonia
- Esophageal Rupture
- Necrosis And Ulcerations Of Esophageal Mucosa
- Obstruction to pharynx.
- Algorithm for the treatment of bleeding from acute esophageal varices.
Management of an Acute Rebleed:
- About 30% of patients develop rebleed within 5 days after a single therapeutic endoscopy.
- Source of the rebleed should be established by endoscopy. Sometimes it is due to an ulcer developed due to previous sclerotherapy and it is difficult to manage.
- Management: Repeat endoscopic therapy once only to control rebleeding and further sclerotherapy or banding should not be done.
Transjugular Intrahepatic Portocaval Shunt (TIPS):
- Indication: When the bleeding does not stop after two sessions of endoscopic therapy within 5 days.
- Technique: A guidewire is passed from the jugular vein into the liver. An expandable metal shunt is forced over it into the liver substance (intrahepatic) to form a portocaval shunt (between the systemic and portal venous systems).
- Advantages: It reduces the hepatic sinusoidal and portal vein pressure without the risks of general anesthesia and major surgery.
- Disadvantages: It is useful only for the short term.
- If the patient does not respond, transjugular intrahepatic portosystemic shunt (TIPSS) is useful in most patients.
Emergency Surgery:
- Indications:
- When other measures fail
- If Tips Is Not Available
- Continued Or Recurrent Hemorrhage
- If the bleeding is from gastric fundal varices.
- Techniques: Esophageal transection and ligation of the feeding vessels to the bleeding varices. Infrequently acute portosystemic shunt surgery and esophageal staple transaction.
Prevention Of Variceal Bleeding—Prophylaxis:
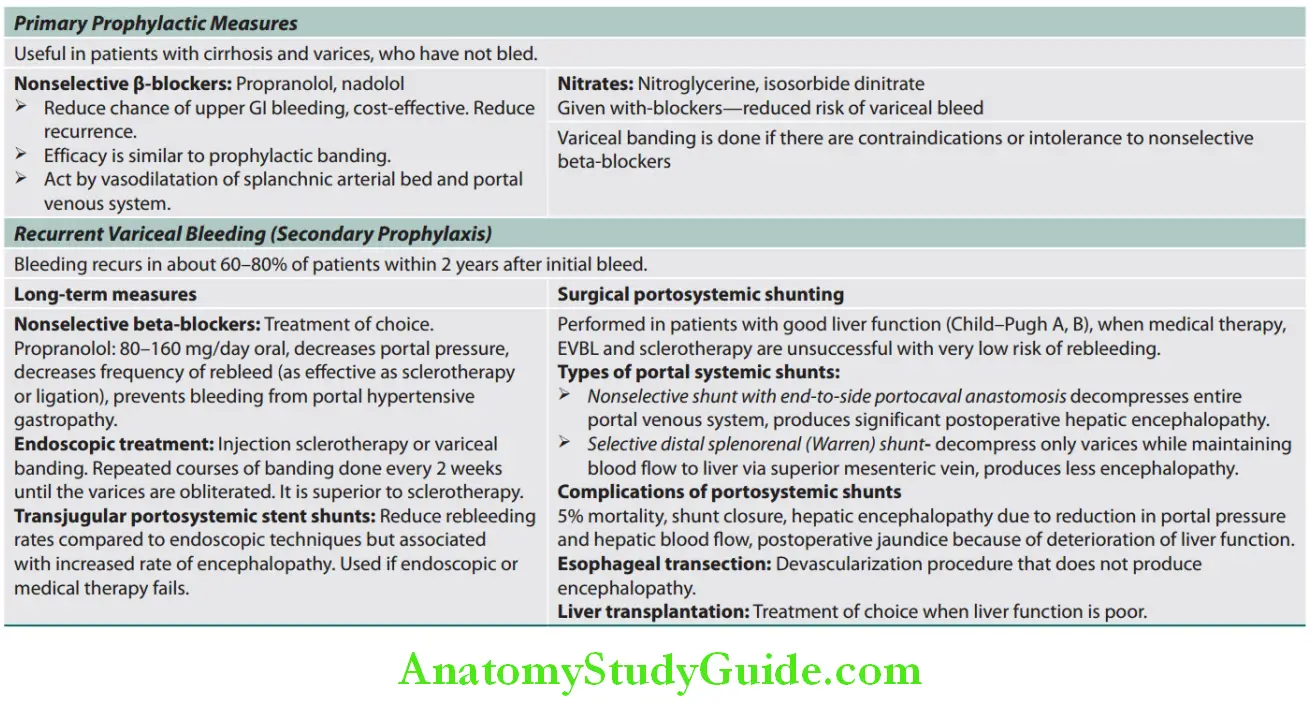
Hepatic Encephalopathy:
Question 47. Define hepatic (portosystemic) encephalopathy.
Answer:
Hepatic Encephalopathy Definition:
Hepatic encephalopathy or portosystemic encephalopathy is defined as a neuropsychiatric syndrome (alteration in mental status and cognitive function) occurring secondary to chronic liver disease. Encephalopathy may be acute and potentially reversible, or chronic and progressive.
Etiology:
Question 48. Discuss the etiology, pathogenesis, clinical features, precipitating factors, investigations, and treatment/management of hepatic encephalopathy.
(or)
Write short essay on alcoholic cirrhosis with acute hepatic encephalopathy.
Answer:
- More common in patients with chronic liver disease such as cirrhosis. In patients with portal hypertension, it develops due to spontaneous “shunting” or in patients following a portosystemic shunt procedure, e.g., TIPS (transjugular intrahepatic portocaval shunt). Adult-onset citrullinemia type II closely mimics hepatic encephalopathy.
- Acute encephalopathy can occur in acute fulminant hepatic failure.
Hepatic encephalopathy Pathogenesis:
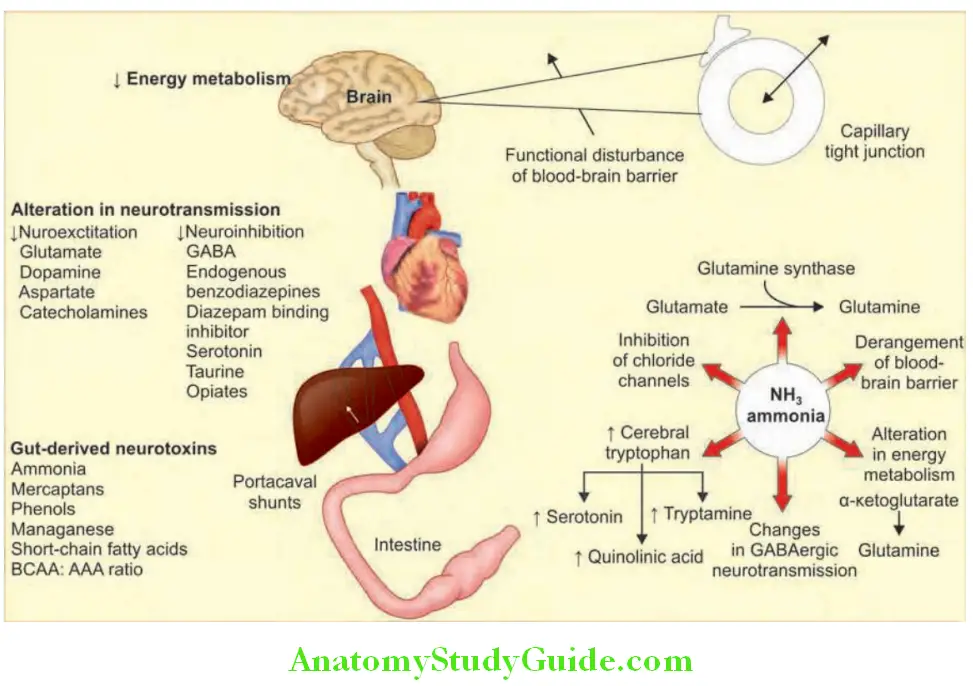
- Mechanism is unknown but many factors play a role. Six pathogenic mechanisms have been proposed.
- Gut-derived neurotoxins that are normally metabolized by the liver
- Brain water homeostasis
- Oxidative/nitrosative stress
- Astrocyte dysregulation
- Neurotransmitter dysfunction (decreased glutamine, increased GABA, serotonin)
- Infection and inflmmation
- Hepatic encephalopathy develops due to disturbance of brain function resulting from various toxic substances reaching the brain. Normally, these toxic substances are derived from the intestine (gut-derived neurotoxins) and carried by portal circulation to the liver, where they are detoxified. Hence, they neither enter the systemic circulation nor reach the brain.
- In hepatic encephalopathy, these toxic substances are not removed by the liver and reach the brain. Three factors are responsible for this are:
- Vascular shunting/bypassing the liver: Th portal blood bypasses the liver via the collaterals into systemic circulation and the “toxic” metabolites pass directly to thebrain.
- Decreased liver mass: It results in severe hepatocellular dysfunction leading to defective detoxifiation of the toxic substances.
- Increased permeability of the blood-brain barrier: Allows the toxins to enter the brain.
- Toxic substances: Involved in hepatic encephalopathy is listed in Ammonia plays a major role and is produced by the breakdown of protein by intestinal bacteria.
Toxic substances involved in hepatic encephalopathy:
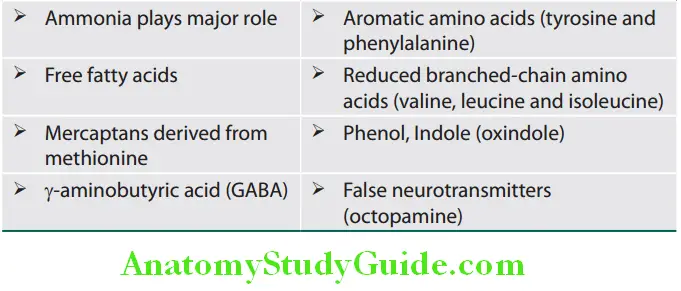
Precipitating Factors for Portosystemic/Hepatic Encephalopathy:
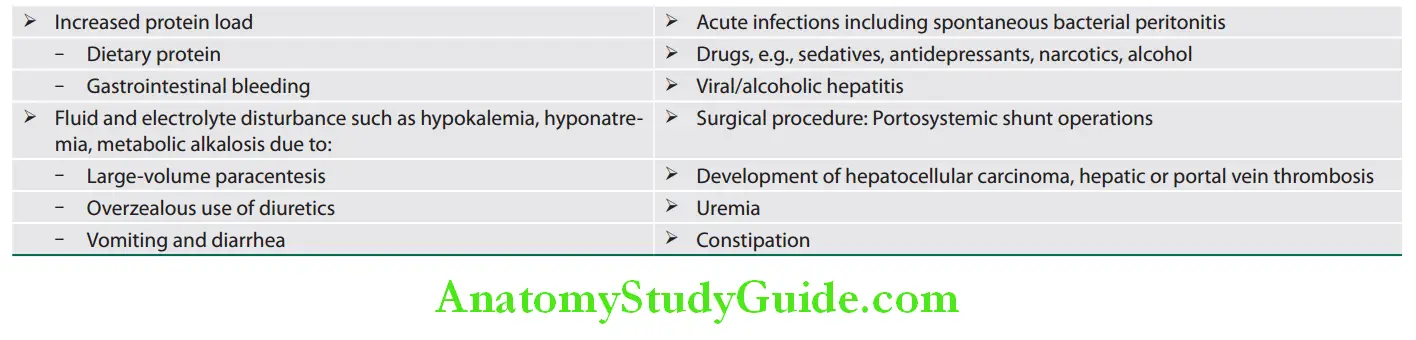
Question 49. List the precipitating causes of hepatic encephalopathy.
Answer:
Types of Hepatic Encephalopathy:
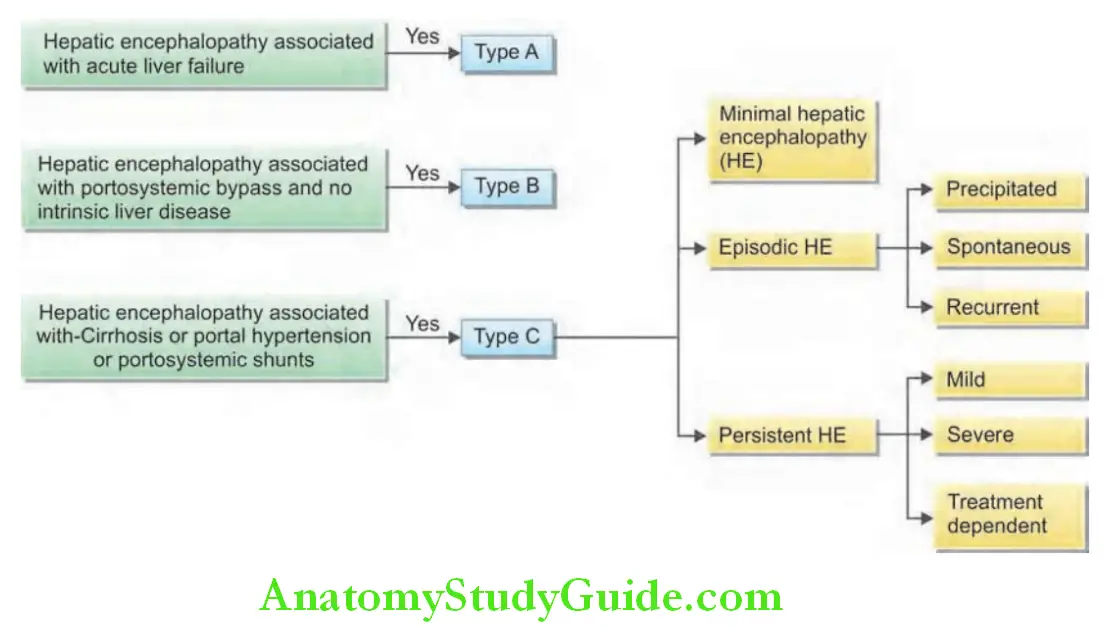
Hepatic Encephalopathy Clinical Features:
- Acute hepatic (portosystemic) encephalopathy often has a precipitating factor and the patient becomes drowsy and comatose within weeks to months. Brain edema may occur with severe encephalopathy and may lead to cerebral herniation.
- Chronic hepatic (portosystemic) encephalopathy
- Disturbances in consciousness and behavior may fluctuate. Hypersomnia is the earliest feature and may progress to reversion of sleep rhythm (daytime somnolence). Patient may become quite violent and difficult to manage or may be very sleepy and difficult to rouse. Patients are irritable, confused, disoriented with slow slurred speech, later may become drowsy and eventually progress to coma.
Clinical grade of hepatic encephalopathy are presented in Table:
West Haven criteria clinical grade of hepatic encephalopathy:
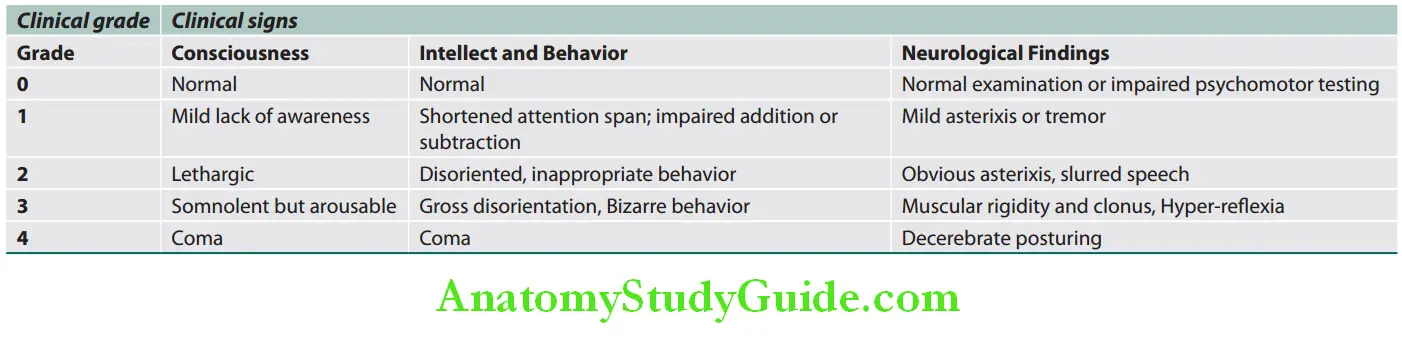
- Change in personality mood and intellect
- General features: Nausea, vomiting, and weakness
Hepatic Encephalopathy Signs:
- Fetor hepaticus (a sweet smell to the breath).
- Asterixis (flapping tremors) (refer Fig. 113A) is a motor disturbance marked by intermittent lapses of an assumed posture, as a result of intermittency of sustained contraction of groups of muscles. Usually manifests as a bilateral flapping tremor at the wrist, metacarpophalangeal, and hip joints. It may also be seen in tongue, foot, and any skeletal muscle.
- Mechanism: Probably due to interruption of the posture pathway in the rostral reticular formation and abnormal joint proprioception.
- The lapse of posture has been termed “negative myoclonus” because during tonic muscle contraction (i.e., posture), a short electromyography (EMG) silent period precedes the tremor.
- Causes: Hepatic encephalopathy, renal failure, metabolic encephalopathy, CO2 toxicity, Wilson’s disease, electrolyte abnormalities (hypoglycemia, hypokalemia, and hypomagnesemia), drug intoxications [e.g., barbiturate intoxication, alcoholism, phenytoin intoxication (“phenytoin flap”) and primidone intoxication] and psychotropic drugs (clozapine, sodium valproate, and risperidone). Lesions in the genu and anterior portion of the internal capsule or ventrolateral thalamus may cause unilateral asterixis.
- Fluctuating neurological signs
- Constructional apraxia with the patient being unable to write or draw
- Hypertonia—later hypotonia
- Hyperreflexia and extensor plantar, later loss of reflexes International Society for Hepatic Encephalopathy and Nitrogen Metabolism (ISHEN) criteria and Full Outline of
Unresponsiveness (FOUR):which assesses four components: eye response, motor response, brain-stem reflex, and respirations are better scoring systems.
Hepatic Encephalopathy Diagnosis: It is based on clinical features. Diagnosis of minimal hepatic encephalopathy is currently based on neuropsychometric tests, including the number connection test, digit symbol test, and the block design test.
Reitan Number Connection Test:
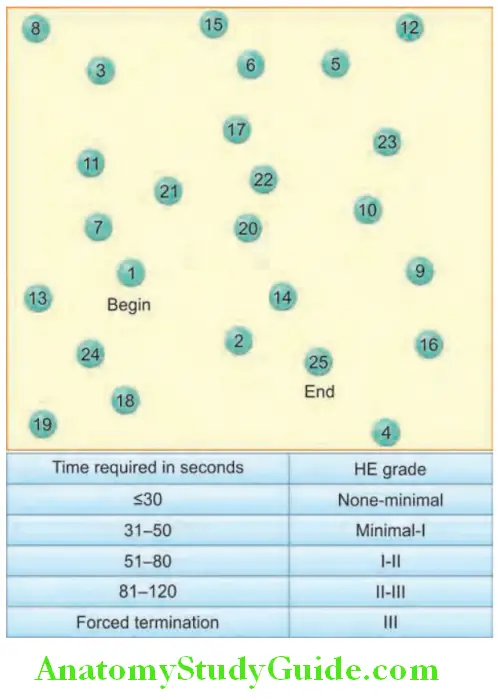
There are 25 numbered circles which can normally be joined together within 30 seconds.
Critical Flicker Frequency:
Critical Flicker frequency (CFF) is a test of retinal gliopathy (that occurs in patients with HE) that can be performed using a portable machine.
During this test, the patient is asked to indicate the maximum frequency at which they can still perceive the light as flickering while changing the frequency over time.
A CFF threshold of 38–39 Hhas been shown to differentiate between manifest HE (i.e., early stages of OHE) and no HE
Hepatic Encephalopathy Investigations:
- Blood ammonia levels: Raised (upper limit of normal is 0.8–1 μg/mL) and can be used for the differential diagnosis of coma and to follow a patient with PSE.
- Serum levels of3-nitrotyrosine may be elevated in patients with minimal hepatic encephalopathy
- Electroencephalography (EEG): Bilaterally synchronous decrease in wave frequency and an increase in wave amplitude, associated with the disappearance of a readily discernible normal alpha-rhythm (8–13 cps).
- Cerebrospinal fluid: Glutamine increased, proteins normal, and cell count normal
- Visual evoked potential abnormalities may be present during subclinical encephalopathy.
- Routine LFTs only confirm the presence of liver disease and not the presence of encephalopathy.
- Imaging is performed to rule out other causes.
Question 50. Write short note on treatment of hepatic encephalopathy.
Answer:
Treatment of hepatic encephalopathy:
Treatment is multifactorial. Restriction of protein intake is reserved for resistant cases.
General measures:
- Management or removal of the precipitating factors is the most important aspect in the treatment.
- Maintain nutrition with adequate calories 35–40 kcal/kg/day.
- Maintain hydration and correct the electrolyte imbalance.
- Protein restriction in the diet. Administer 0–2 g/kg of proteins daily, preferably vegetable protein.
- Zinc supplementation may be helpful and is relatively harmless.
- Stop or reduce diuretic therapy.
- Treat any infection with suitable antibiotics (e.g., ampicillin, rifaximin, metronidazole, or neomycin).
- Stop alcohol. Avoid sedatives. For restlessness and excitement, small dose of diazepam or midazolam may be given intravenously.
Evacuation of the bowels and sterilizing the bowel:
- Give purgation and enemas to empty the bowels of nitrogenous substances.
- Lactulose therapy: To reduce plasma ammonia level
- Actions: Lactulose (beta-galactosidofructose) is a nonabsorbable disaccharide, which acts as an osmotic purgative. In the colon, lactulose and lactitol are catabolized by the bacterial flora to lactic acid and acetic acid. It lowers the colonic pH and favors the formation of the nonabsorbable NH + from NH, trapping NH + in the colon and thus reducing plasma ammonia concentrations. Other mechanisms of action include
- Increased incorporation of ammonia by bacteria for synthesis of nitrogenous compounds,
- Modification of colonic flora, resulting in displacement of urease-producing bacteria with nonurease-producing bacteria and cathartic effects that improves GI transit, allowing less time for ammonia absorption,
- Increased fecal nitrogen excretion due to the increase in stool volume
- Reduced formation of toxic short-chain fatty acids (e.g., propionate, butyrate, valerate).
- Dose: 15–30 mL three times orally per day. Dose is increased gradually till there are two to three loose stools per day.
- Lactitol (β-galactoside sorbitol 30 g daily): It has a similar action, more palatable and better than lactulose.
- Poorly absorbed antibiotics: They are often used as adjunctive to sterilize the gut in patients who have difficulty with lactulose. They reduce the intestinal ammonia production by bacteria.
- Alternating administration of neomycin and metronidazole to reduce the individual side effects of each neomycin for nephrotoxicity and ototoxicity and metronidazole for peripheral neuropathy.
- Rifaximin semisynthetic, gut-selective, and nonabsorbable oral antibiotic, derived from rifamycin and a structural analog of rifampin in the dose of 550 mg twice daily or 400 mg thrice daily is very effective and without any side effects of neomycin or metronidazole. It has only 0.4% systemic absorption.
- Poorly absorbed antibiotics: They are often used as adjunctive to sterilize the gut in patients who have difficulty with lactulose. They reduce the intestinal ammonia production by bacteria.
- When there is GI bleeding, Ryle’s tube aspiration and bowel washes are performed to remove the blood and blood products. It reduces the production of nitrogen in the gut.
- Other drugs tried are:
- Bromocriptine
- L-ornithine L-aspartate (LOLA)
- Branched-chain amino acids
- Probiotics and prebiotics
- Sodium benzoate
- Zinc, polyethylene glycol, acarbose
- Flumazenil
- Melatonin
- Novel treatment strategies include use of L carnitine, rivastigmine, endocannabinoids, and mGluR1 antagonists.
- In acute liver failure, mannitol and judicious use of intravenous fluids to reduce the spontaneous cerebral edema (controversial).
- Liver transplantation.
- MARS—Molecular Adsorption Reversibility System—purifies the blood by removal of albumin bound as well as water-soluble substrates.
Management of hepatic encephalopathy is summarized in Flowchart:
Management of hepatic encephalopathy:
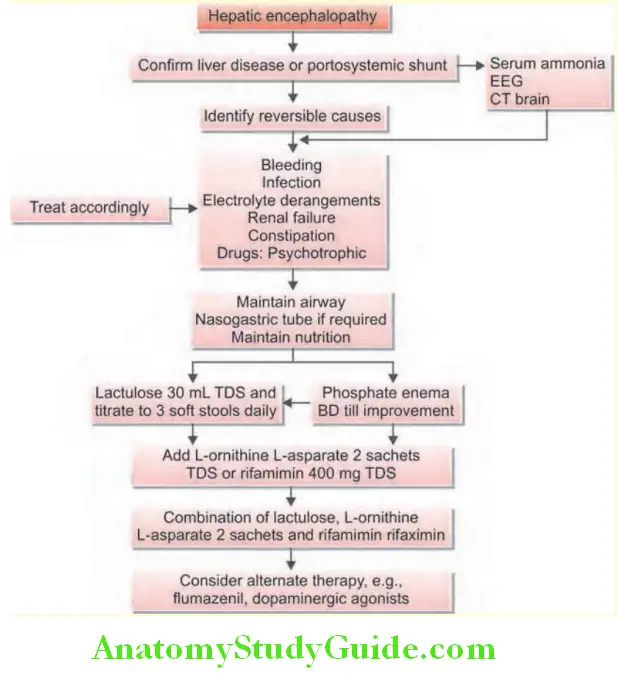
Hepatorenal Syndrome:
Question 51. Write a short note on hepatorenal syndrome.
Answer:
- Hepatorenal syndrome (HRS) is a form of functional azotemia without renal pathology in patients with advanced cirrhosis or acute liver failure.
- The urine output is low, tubular function is normal, and kidneys are histologically normal.
- This occurs in 10% of patients with advanced cirrhosis with jaundice and ascites.
Pathogenesis:
- Initially, severe peripheral vasodilatation (probably due to nitric oxide), leads to severe reduction in the effective blood volume and hypotension.
- This activates the homeostatic mechanisms and rennin-angiotensin-aldosterone system leading to vasoconstriction of the renal vessels.
- Increased preglomerular vascular resistance directs the flow of blood away from the renal cortex. This leads to a reduced glomerular filtration rate.
- Eicosanoids are another mediator involved in pathogenesis of HRS.
Precipitating Factors for HRS:
Precipitating factors for hepatorenal syndrome (HRS):
- Gastrointestinal bleeding
- Aggressive paracentesis
- Diuretic therapy
- Sepsis including spontaneous bacterial peritonitis
- Diarrhea
Hepatorenal Syndrome Clinical Types:
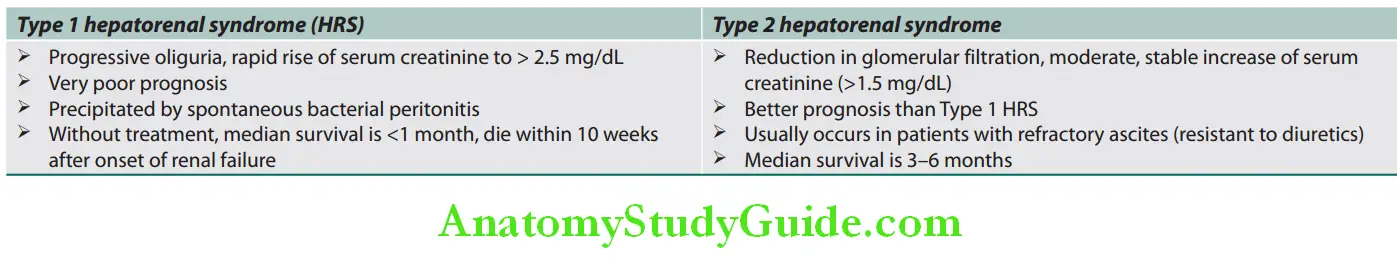
Hepatorenal Syndrome Clinical Features:
- Develops in advanced cirrhosis, almost always with ascites
- Anorexia, weakness, fatigue, oliguria, nausea, vomiting, and thirst
- Terminally coma deepens and hypotension develops.
Hepatorenal Syndrome Investigations:
- Urea and creatinine levels: High.
- Serum sodium: Less than 130 mEq/L.
- Urine sodium excretion: Less than 10 mEq/day
- Urinalysis: Normal
- Urine: Plasma osmolality ratio is more than 5.
Hepatorenal Syndrome Diagnosis:
- Usually made in the presence of a large amount of ascites in patients who have a stepwise progressive increase in creatinine.
- Diagnostic criteria HRS.
All of the following must be present for the diagnosis of hepatorenal syndrome (HRS):
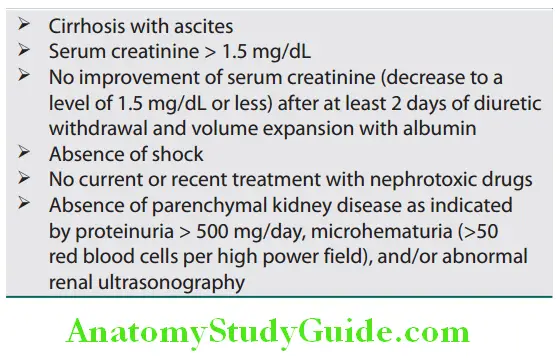
Hepatorenal Syndrome Treatment:
- Prevention:
- Avoid over vigorous diuretic therapy
- Slow treatment of ascites
- Early recognition of electrolyte imbalance, hemorrhage, or infection
- Stop diuretic therapy:
- Correct hypovolemia by intravenous plasma protein solution or salt-poor albumin
- Screen and treat infection including SBP
- An albumin infusion (1 g/kg bodyweight/day up to a maximum of 100 g/day) in combination with terlipressin (vasopressin analogs) is effective short-term medical therapy
- Currently, midodrine (α-agonist) along with octreotide and intravenous albumin is also used.
- Renal replacement therapy
- TIPSS if vasoconstrictors fail
Liver transplantation is the treatment of choice
Ascites:
Question 52. Discuss the pathogenesis and management of ascites and refractory ascites in cirrhosis.
(or)
Discuss the definition, mechanism, causes, clinical features and differential diagnosis of ascites.
Answer:
Ascites is defined as the accumulation of excess fluid within the peritoneal cavity.
The most common (85% of cases) cause of ascites is portal hypertension caused by cirrhosis; however, malignant or infectious causes can also produce ascites.
Pathogenesis/Mechanisms:
Pathogenesis of Ascites in Cirrhosis:
It is complex, involving the following mechanisms:
- Portal hypertension: Increase in portal vein hydrostatic\ pressure and results in extravasation of fluid from plasma into the peritoneal cavity.
- Hypoalbuminemia: Due to decreased synthetic function in a cirrhotic liver, plasma oncotic pressure is reduced. This results in an extravasation of fluid (ascites and edema).
- Splanchnic vasodilation and hyperdynamic circulation. It reduces systemic arterial blood pressure, activates reninangiotensin-aldosterone system with the development of secondary hyperaldosteronism. Failure of liver to metabolize aldosterone intensifies secondary hyperaldosteronism. It leads to sodium retention and fluid accumulation and expansion of the extracellular fluid volume. The combination of portal hypertension, splanchnic arterial vasodilation, and sodium and water retention increases the hydrostatic pressure as well as permeability of interstitial capillaries. It causes extravasation of fluid into the peritoneal cavity.
- Percolation of hepatic lymph into the peritoneal cavity: In cirrhosis, hepatic lymphatic flow exceeds thoracic duct capacity. The excess lymph oozes freely from the surface of cirrhotic liver into the peritoneal cavity and causes ascites.
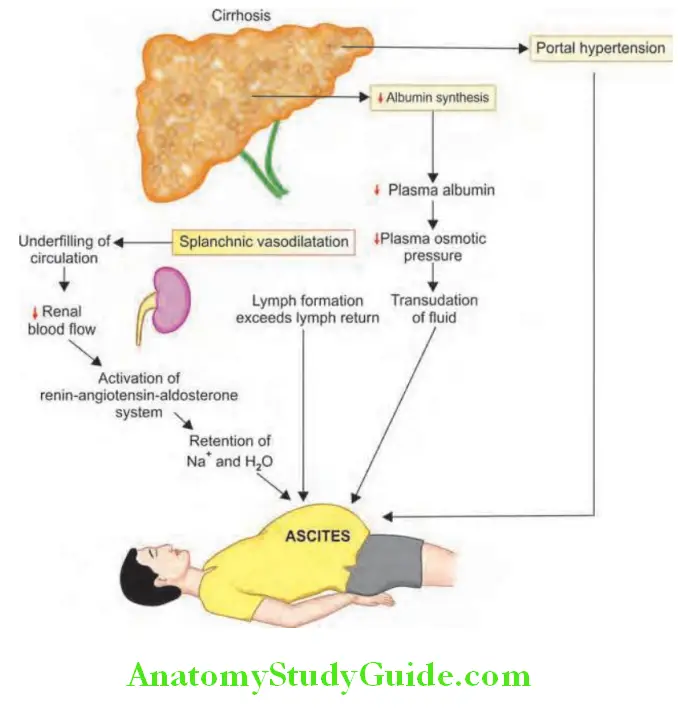
Theories of ascites: According to the underfill theory, transudation from the liver leads to reduction of the blood volume, thereby stimulating sodium (Na) retention by the kidney.
- According to the overflow theory, increased portal pressure stimulates renal Na retention through incompletely defined mechanisms.
- The vasodilation theory suggests that portal hypertension leads to vasodilation and relative arterial hypotension.
Pathogenesis in the Absence of Cirrhosis:

Ascites Clinical Features:
Symptoms:
- Distension of abdominal (increase in abdominal girth) and bloated feeling often accompanied by peripheral edema.
- Ascitic fluid may cause elevation of diaphragm and compromise respiratory function and produce dyspnea and orthopnea.
- Indigestion and heart burns due to gastroesophageal reflux may develop because of increased intra-abdominal pressure.
- Malnourishment and patients have muscle wasting and excessive fatigue and weakness.
Ascites Signs:
- Distension of abdomen and fullness of flanks (bloated feeling)
- Umbilicus appear flat or everted.
- Skin over the abdominal wall appear stretched and shiny.
- In the erect posture, hypogastrium appears prominent.
- Abdominal wall may show two types of distended veins:
- Caput medusae in which veins radiate out from the umbilicus with blood flow away from the umbilicus. They represent collaterals developed due to portal hypertension.
Five signs of ascites:
- Horseshoe dullness
- Shifting dullness
- Puddle sign
- Fullness of flank
- Fluid thrill/fluid wave
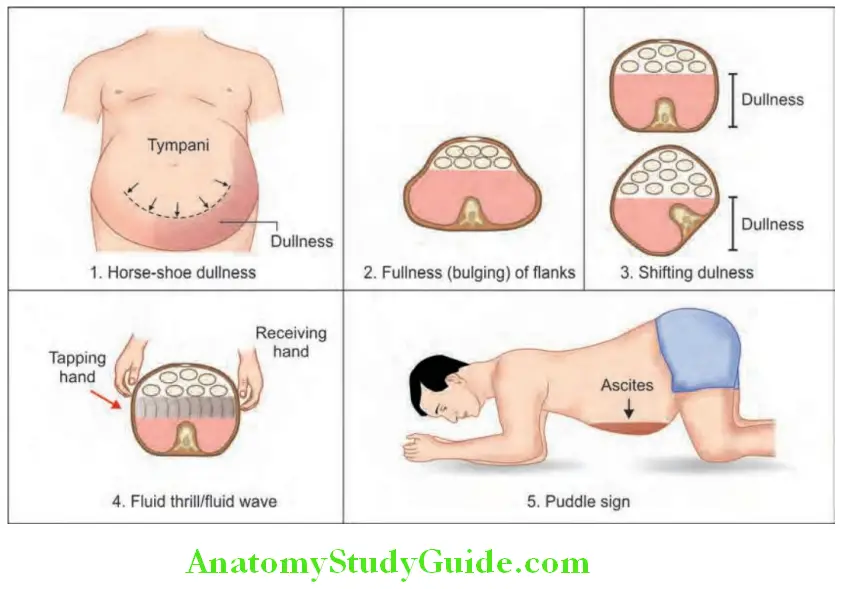
-
- Prominent veins in the flanks with blood flow from below upward. They represent the IVC collaterals developed due to compression of IVC by severe ascites.
- Abdominal dullness: It indicates the presence of fluid in the abdominal cavity.
- Dullness in the paraumbilical zone is detected with smaller amounts of fluid. This is elicited by asking the patient to assume knee elbow position and paraumbilical region percussed. Normally, the percussion note is tympanitic. With mild ascites the dullness is found only in the flanks.
Question 53. What is Puddle sign?
Answer:
- Puddle sign/Lawson’s sign: It can detect even ascites as low as 120 mL. Patient is asked to lie in prone position for 5 minutes, followed by knee elbow position. Place the diaphragm of the stethoscope on the most dependent part of the abdomen. Repeatedly flick one flank lightly. Diaphragm is gradually moved to the opposite flank. A marked change in the intensity and character of percussion note indicates the presence of fluid.
- With moderate ascites, both the flanks and hypogastric areas are dull (horse shoe-shaped dullness). The epigastrium and
- Umbilical regions show resonant note due to floating intestines.
- With massive ascites, the whole of the abdomen is dull, except for a small region over the umbilicus.
- For elicitation, shifting dullness at least of 500–1000 mL of fluid is required.
- Fluid thrill can be elicited in tense ascites (fluid more than 2,000 mL).
Secondary effcts:
- Edema of scrotum.
- Pedal edema: Due to hypoproteinemia and a functional block of IVC caused by tense ascites.
- Pleural effusion: Mainly on the right side. Pleural effusion is due to defects in the diaphragm that allows the ascitic fluid to pass into the pleural cavity.
- Cardiac apex: Shifted upward because of raised diaphragm
- Distension of neck veins: Secondary to a raised right atrial pressure, which follows tense ascites and raised diaphragm.
- Meralgia paresthetica: It can develop due to compression of lateral cutaneous nerve of thigh.
- Divarication of recti and hernia
Puddle sign Investigations:
Ultrasonography: It is very sensitive and can detect even small amounts of ascitic fluid and also useful in identifying the cause.
- Paracentesis and evaluation of ascitic fluid
- Laparoscopy and biopsy of peritoneum
Aspirate about 10–20 mL of ascitic fluid and ascitic tap the following tests are performed:
- Cell count: A neutrophil count above 250 cells/mm3 usually indicates SBP.
- Gram stain and culture: For bacteria and acid-fast bacilli.
- Protein: Total ascitic fluid protein less than 5 g/dL indicates an increased risk of SBP.
- SAAG (Serum ascites albumin gradient): It is the differences between serum albumin and ascitic fluid albumin. SAAG is useful for differentiating ascites caused by portal hypertension from nonportal hypertensive ascites. It is calculated by subtracting the ascitic albumin concentration form the serum albumin concentration and does not change with diuresis. It is better indicator than simple estimation of protein in the ascitic fluid. SAAG is not a ratio, it is the difference. Classification of ascites based on SAAG is presented in Table 138. Corrected SAAG = Uncorrected SAAG × 0.16 × (Serum globulin g/dL + 5).
- Cytology: For malignant cells to exclude neoplasms causing ascites
- Amylase: To exclude pancreatic ascites. It is increased in acute pancreatitis.
Ascitic fluid changes in cirrhosis are presented. Causes of high- and low-serum-ascites albumin gradient.
Causes of high and low serum-ascites albumin gradient:
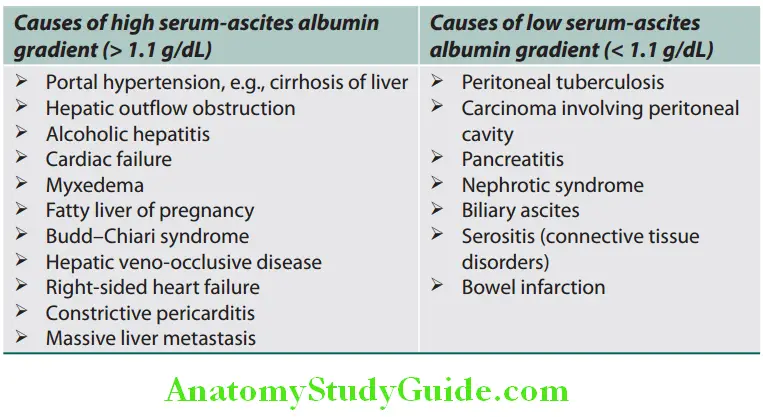
Ascitic flid changes in cirrhosis:
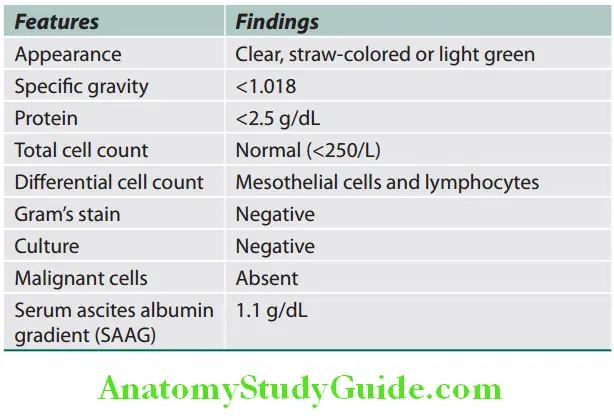
Diffrential Diagnosis of Ascites:
Nature of Ascitic Fluid:
The ascitic fluid may be transudate or exudate.
Differences between transudates and exudates are listed in Table:
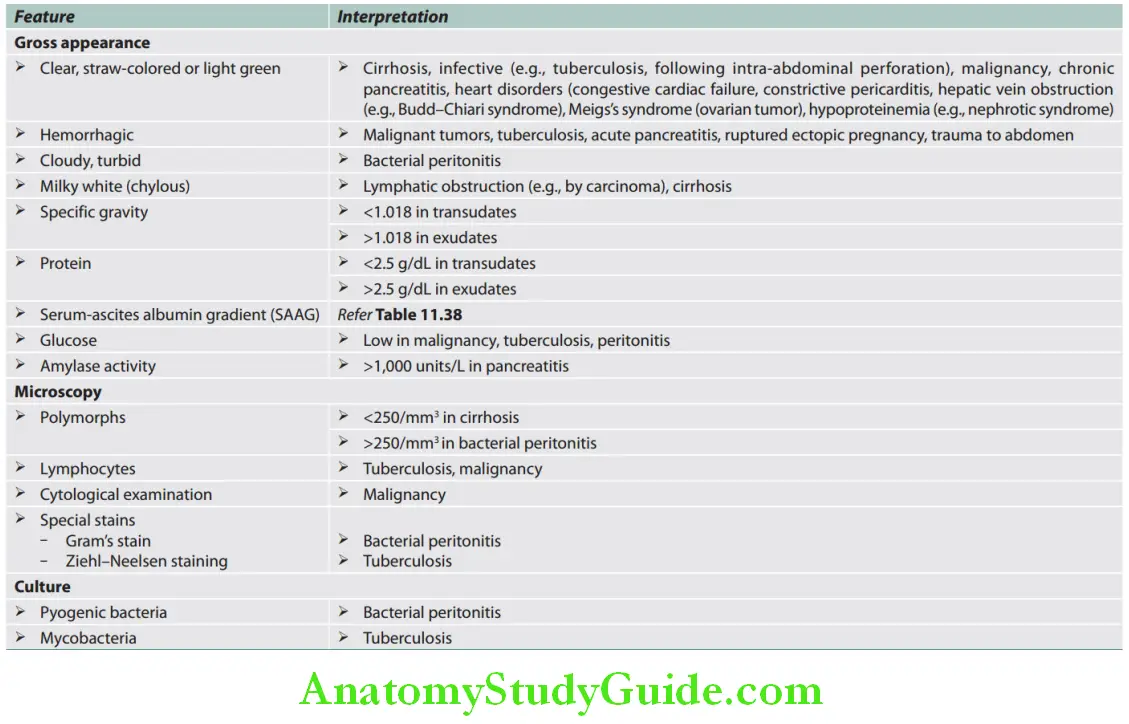
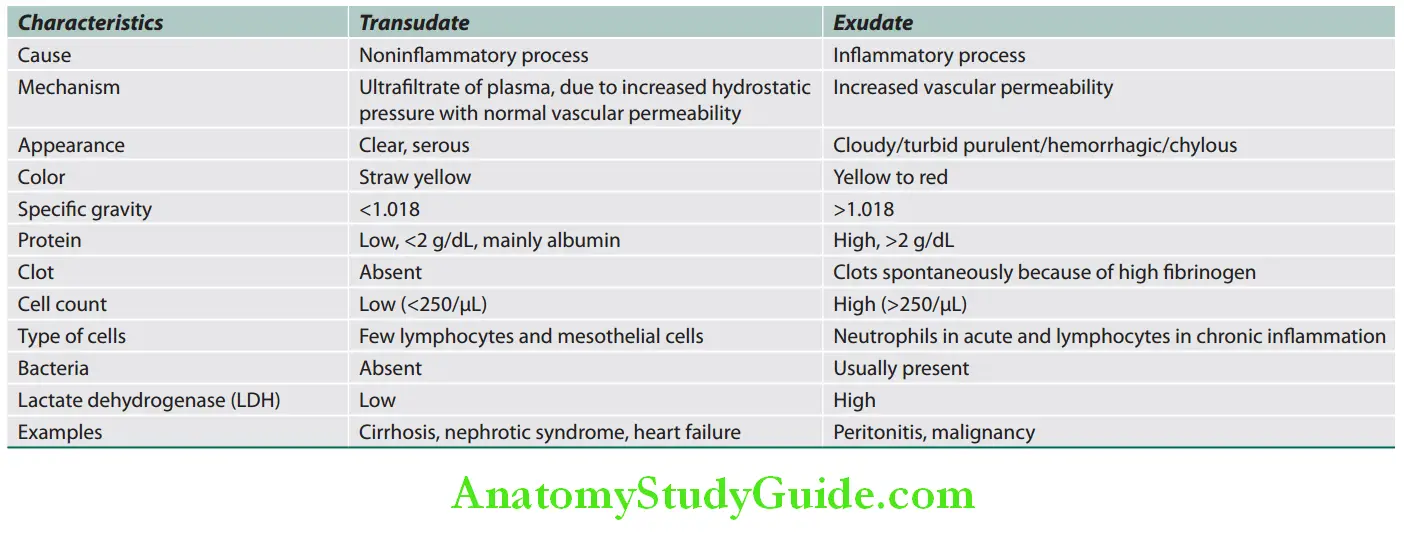
Puddle sign Causes: Various causes of ascites are listed in Table:
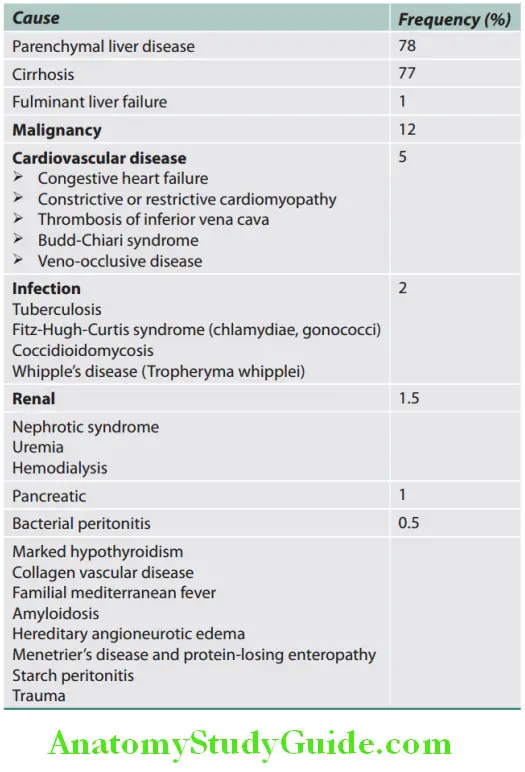
Puddle sign Management:
- General measures:
- Hospitalization is necessary, if there is massive ascites.
- Check serum electrolytes, renal function tests at the start of treatment and twice a week.
- Weigh the patient daily. Measure abdominal girth and strict intake and output monitoring daily.
- Bed rest alone induces diuresis in a small proportion of people because renal blood flow increases in the horizontal position, but in practice is not helpful. Ascites treatment algorithm is shown in Flowchart.
- Dietary restriction of sodium by reducing sodium intake to 40 mmol in 24 hours and maintain an adequate protein and calorie intake with a palatable diet. First-line treatment of patients with cirrhosis and ascites consists of sodium restriction (88 mmol/day [2000 mg/day]) and diuretics (oral spironolactone with or without oral furosemide).
- Fluid restriction is not necessary unless serum sodium is less than 125 mmol/L
- Drugs: Many contain significant amounts of sodium (up to 50 mmol daily). Examples include antacids, antibiotics (particularly the penicillins and cephalosporins) and effervescent tablets. Sodiumretaining drugs (nonsteroidal, corticosteroids) should be avoided. Fluid restriction to 1,000–1,500 mL/day is necessary if the serum sodium is under 128 mmol/L.
- Diuretics
- Aim of diuretic therapy: To produce a net loss of fluid of about 700 mL/day (0.7 kg weight loss in patients with ascites alone or
- 1.0 kg if both ascites and peripheral edema is present). The maximum rate at which ascites can be mobilized is 500–700 mL/day. This is to prevent diuretic-induced renal failure and/or hypernatremia.
- Diuretics are administered in a step-wise manner.
- Aldosterone antagonists: As there is secondary hyperaldosteronism, the diuretic of first choice is the one of the aldosterone antagonists (potassium-sparing diuretics), e.g., spironolactone, triamterene, amiloride. Spironolactone is started at a low dose of 25 mg QID (100 mg daily), and gradually stepped up every week to a maximum of 400 mg/day (providing there is no hyperkalemia).
- Chronic administration of spironolactone produces gynecomastia. Eplerenone 25 mg once daily does not produce gynecomastia.
- Loop diuretics: When a large dose of spironolactone has failed, add a loop diuretic, such as furosemide 20–40 mg or bumetanide 0.5 mg or 1 mg daily. Usually spironolactone is combined with furosemide. Disadvantages of loop diuretics include development of hyponatremia, hypokalemia, and volume depletion.
- Stop all diuretics, if severe hyponatremia (sodium <120 mEq/L), progressive renal failure or worsening of hepatic encephalopathy occurs. Vaptans may improve serum sodium in patients with cirrhosis and ascites.
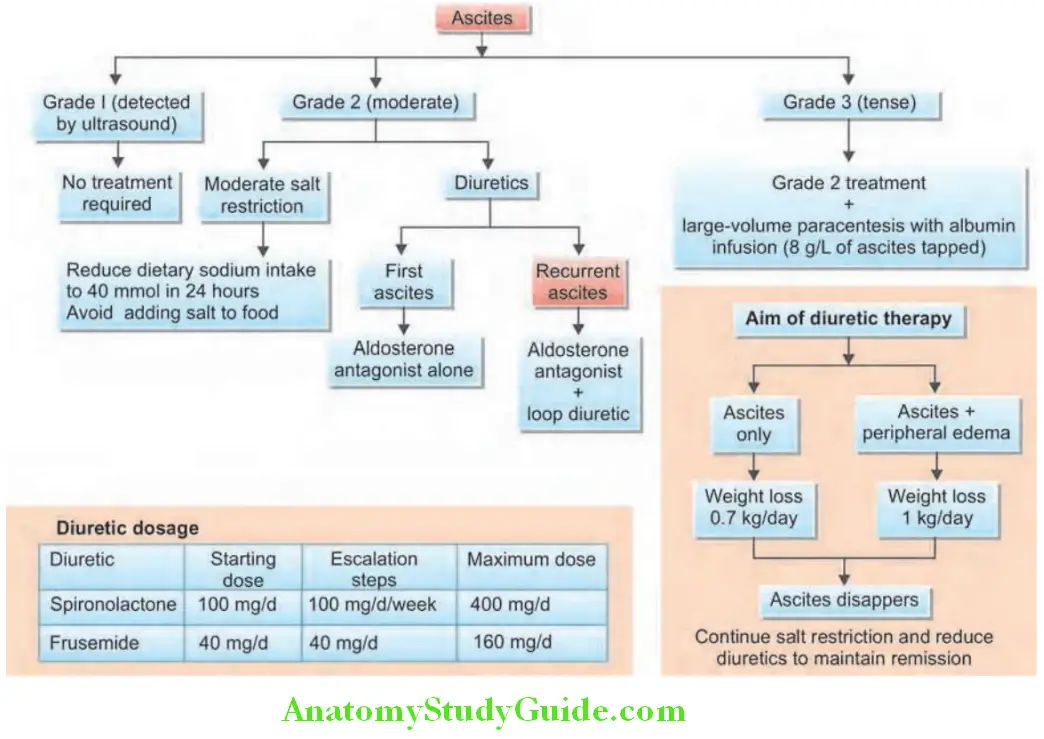
Treatment of Refractory Ascites:
Question 54. Write short essay/note on indication, procedure, and complications of abdominal paracentesis.
Answer:
Refractory ascites is defined as fluid overload that
- Is unresponsive to sodium-restricted diet and high-dose diuretic treatment (400 mg per day of spironolactone and 160 mg per day furosemide)
- Recurs rapidly after therapeutic paracentesis.
They are managed on the following lines:
- Intravenous salt-poor albumin, 25 g in 3 hours.
- Oral midodrine 7.5 mg three times daily.
- Large-volume paracentesis
- Indications
- Procedure:
- Remove 3–5 L of fluid over 1–2 hours.
- Salt-free albumin (8 g/L of ascetic fluid removed) if volume is more than 4–5 L is removed. If albumin is not available, dextran-70 may be used. Strict monitoring is necessary.
- Complication: Hypovolemic and renal dysfunction (postparacentesis circulatory dysfunction) more likely with removal of > 5 L and worse liver function.
- Shunts:
- TIPSS: It is used for resistant ascites, if there is no spontaneous portosystemic encephalopathy and no disturbance of renal function.
- Le Veen shunt: It is a peritoneovenous shunt that allows the peritoneal fluid to drain directly into the internal jugular vein.
- Slow low-dose continuous albumin, Furosemide with or without Terlipressin SIFA(T)infusion.
- Liver transplantation.
Indications for large-volume paracentesis:
- Refractory ascites
- Used to relieve symptomatic tense ascites, e.g., cardiorespiratory distress due to gross ascites.
- Impending rupture of a hernia.
Bacterial Peritonitis:
Bacterial Peritonitis Types:
- Acute bacterial peritonitis
- Chronic bacterial peritonitis.
1. Acute Bacterial Peritonitis:
Causes: Appendicitis, perforated peptic ulcer or typhoid ulcer, cholecystitis, diverticulitis, gangrene of the small intestine and ulcerative colitis.
Symptoms:
Fever, nausea, thirst, vomiting, severe abdominal pain. No flatus.
Bacterial Peritonitis Signs:
- Hippocratic facies
- Tachycardia, hypotension, and shock
- Board-like rigidity, tenderness, and rebound tenderness
- Absent peristaltic sounds.
Bacterial Peritonitis Investigations:
Investigations in acute bacterial peritonitis:
- Plain radiograph of abdomen:
- Dilated loops of intestine
- Gas under the diaphragm if there is intestinal perforation
- Ascitic fluid:
- Exudate, turbid, or purulent
- Shows intestinal contents in the fluid
- Cell counts: Markedly increased, polymorphs > 250/mm 3
- Gram stain: Positive for bacteria
- Culture: It shows growth of organism.
2. Chronic Bacterial Peritonitis:
- Associated with subacute intestinal obstruction
- Ascitic fluid: Exudate, protein is high, and contains a large number of chronic inflammatory cells.
- Classification of ascitic fluid infections is listed in Table.
Treatment of the underlying cause.
Spontaneous Bacterial Peritonitis:
Question 55. Write short note on diagnosis and management of spontaneous bacterial peritonitis.
Answer:
Patients with cirrhosis and ascites are highly susceptible to infection of ascitic fluid.
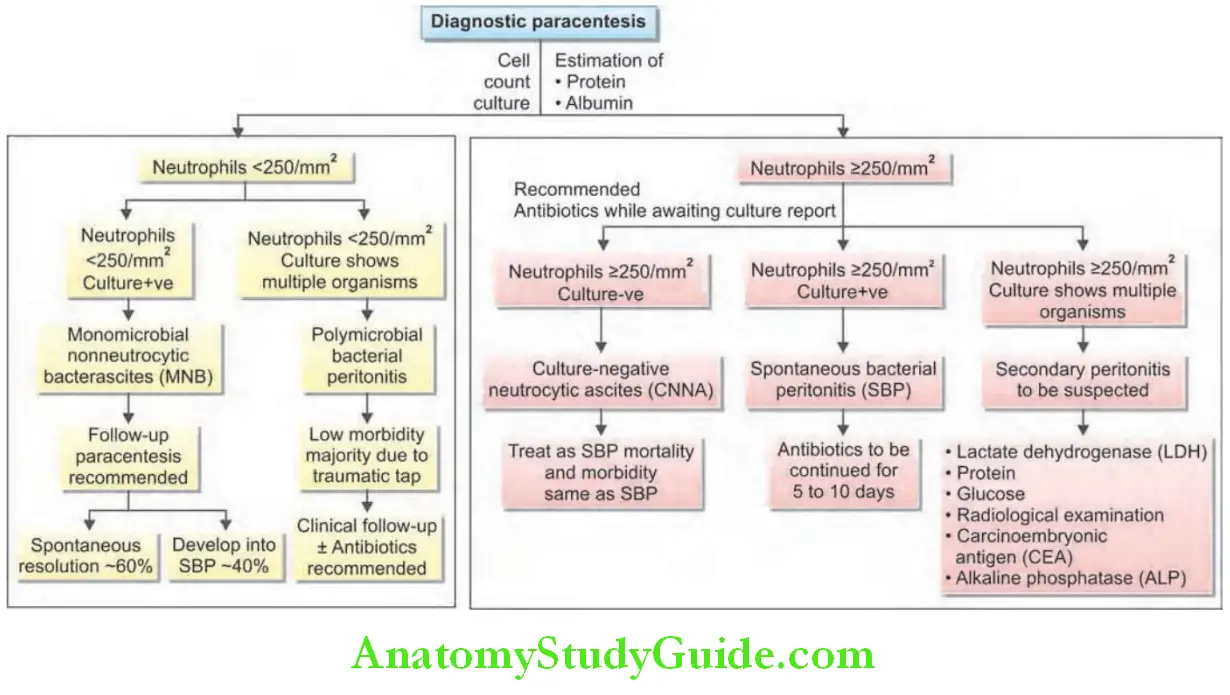
Spontaneous Bacterial Peritonitis Definition:
Spontaneous bacterial peritonitis is a common and severe complication of ascites characterized by spontaneous infection of ascitic without an evident intra-abdominal surgically treatable source.
Spontaneous Bacterial Peritonitis Pathogenesis:
- Causative agents: Most common organisms are Escherichia coli, Klebsiella, or enterococci, or other gut bacteria. Others include streptococci and enterococci.
- Route of infection: The infecting organisms in the gut flora traverse the intestine into mesenteric lymph nodes, leading to bacteremia and seeding of the ascitic fluid by hematogenous spread.
Spontaneous Bacterial Peritonitis Clinical Features:
- SBP should be suspected in any patient with ascites who clinically deteriorates.
- It may present as sudden deterioration or hepatic encephalopathy in a cirrhotic patient with ascites.
- Features include fever, abdominal pain or discomfort and rebound abdominal tenderness or they may present without any of these features.
- In terms of important predictors for identifying cirrhotic patients at greatest risk for SBP, both a high serum bilirubin (above 5 mg/dL) and a low ascitic fluid protein concentration (less than 0 g/dL) have been shown to be independent factors for both initial episodes of SBP as well as for recurrence.
Spontaneous Bacterial Peritonitis Investigations:
- Peripheral blood: Leukocytosis.
- Ascitic fluid:
- Cloudy fluid
- Leukocyte count: More than 500/mmA raised neutrophil count of > 250/mm3 in ascites is alone sufficient for diagnosis and to start treatment immediately.
- pH: Less than 7.
- Culture: Positive. Monomicrobial E. coli is the most common organism.
- Investigations to rule out abdominal sources of peritonitis by imaging
Spontaneous Bacterial Peritonitis Diagnosis:
With clinical suspicion, a diagnostic aspiration (paracentesis) should always be performed in patients with GI bleeding, shock, fever, worsening liver and/or renal function, and hepatic encephalopathy for making the diagnosis.
Spontaneous Bacterial Peritonitis Treatment:
- Third-generation cephalosporin: Such as cefotaxime or ceftazidime being the most commonly used antibiotic. This is modified on the basis of culture results. Dose of cefotaxime 2 g IV 8 hourly for 5 days.
- Alternative therapy in patients without shock or hepatic encephalopathy
- Amoxicillin/clavulanate (2 g IV 8 hourly followed by 625 mg orally)
- Ciprofloxacin (200 mg IV12 hourly followed by 500 mg BID orally)
- Ofloxacin (400 mg twice daily).
- Antibiotic therapy and albumin (5 g/kg bodyweight within 6 hours of detection and 1 g/kg on day 3) reduces risk of type1 HRS.
Prophylaxis for SBP:
Recurrence is common (70% within a year) and prophylaxis is indicated.
Drugs:
- In patients with upper GI hemorrhage: cefotaxime or norfloxacin
- (400 mg BID for 7 days).
- In patients with low ascitic protein content or previous episode of SBP: Long-term quinolones such as norfloxacin (400 mg/day lifelong).
- Alternative but less effective drugs: Include cotrimoxazole (800 mg sulfamethoxazole + 160 mg trimethoprim once a day) or ciprofloxacin (750 mg once a week).
Indications for prophylaxis for spontaneous bacterial peritonitis:
- Patients with acute gastrointestinal bleeding (antibiotic prophylaxis also reduces the rate of rebleeding)
- Patients with a previous episode(s) of SBP and recovered
- Patients with low total ascites protein content < 5 g/dL or severe liver disease and no prior history of SBP
Tuberculous Peritonitis:
- Pathogenesis: Tuberculosis spreads to involve peritoneum by or more of the following sources:
- Through hematogenous seeding of peritoneum
- Through lymphatics or mesenteric nodes
- From genitourinary source
Spontaneous Bacterial Peritonitis Clinical Features:
- Night sweats, weight loss, loss of appetite, malaise, and evening rise of temperature
- Gradual abdominal distension and “doughy feel” of the abdomen.
- Multiple palpable masses in the abdomen produced due to matted omentum and loops of intestine.
Spontaneous Bacterial Peritonitis Investigations:
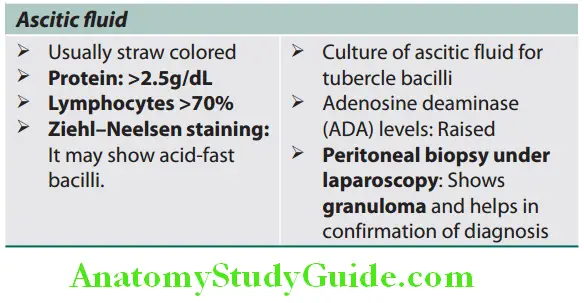
Spontaneous Bacterial Peritonitis Treatment: Antituberculous chemotherapy.
Malignant Ascites:
Malignant Ascites Causes:
- Primary tumors of stomach, colon, ovary or other intra-abdominal tumors may produce exudation fluid into the peritoneal cavity.
- Peritoneal metastasis: From any primary.
- Periumbilical subcutaneous metastatic tumor deposits (known
“Sister-Joseph’s” nodules’), e.g., carcinoma of stomach.
Malignant Ascites Investigations:
- Ascitic fluid.
- Biopsy of the peritoneum by laparoscope: It shows the tumor and useful for confirmation of diagnosis.
Ascitic fluid findings in malignant ascites:
- Appearance: Exudate and hemorrhagic
- Protein: High
- Specific gravity: High
- Microscopy: Sediment shows malignant cells.
Treatment Palliative:
- Repeated paracentesis.
- Intraperitoneal instillation of chemotherapeutic drugs: It may reduce rate of reaccumulation of ascites. The drugs include methotrexate, nitrogen mustard, or immunotherapy (interferon alfa).
Meigs’s Syndrome:
Question 56. Write short note on Meigs’s syndrome.
Answer:
Features of Meigs’s syndrome are presented:
Features of Meigs’s syndrome:
- Pleural effusion is most commonly right sided, may be exudate or transudate.
- Ascites
- Pelvic tumor (commonly fibroma of the ovary) in females. Both ascites and pleural effusion resolve following excision of the pelvic tumor.
Chylous Ascites:
- Characterized by the presence of chyle (intestinal lymph) in the peritoneal cavity. Ascitic fluid is milky or creamy due to the presence of chylomicrons.
- Causes:
- Injury/trauma to the main lymph ducts in the abdomen.
- Malignancy (e.g., lymphoma) or tuberculosis causing obstruction of the intestinal lymphatics
- Filariasis
- Intestinal obstruction associated with rupture of a major lymphatic channel
- Congenital lymph angiectasia
- Clinical features:
- Acute abdominal pain with signs of peritoneal irritation
- Distended, nontender, fluid filling the abdomen (chylous ascites).
- Ascitic fluid:
- Milky or creamy
- High fat (triglycerides > 1,000 mg/dL) content.
- Sudan III staining demonstrates fat globules.
Ascites Praecox:
Question 57. What is ascites praecox?
Answer:
- In ascites praecox, ascites occurs early and is disproportionately prominent as well as appears before the edema. It occurs in constrictive pericarditis, acute Budd–Chiari syndrome, tuberculous peritonitis, and malignant peritonitis.
- In constrictive pericarditis, ascites appears first, followed by edema. This sequence is one of the cardinal features, distinguishing ascites from congestive heart failure in which edema appears first and ascites much later. Edema is minimal in constrictive pericarditis and occurs in the later part of the disease.
Drug And Toxin-Induced Hepatitis:
Question 58. Write a short note on drug-induced liver injury.
Answer:
Types of drug-induced liver injury (DILI):
DILI can also be classified as:
- Immune mediated (allergic)
- Non-immune mediated (non-allergic)
Drug And Toxin-Induced Hepatitis Investigations:
Case defiitions for DILI:
- ≥5 × ULN elevation of ALT
- ≥2 × ULN elevation in ALP (particularly with accompanying elevation in concentrations of GGT in the absence of bone pathology driving the rise in ALP).
- ≥3 × ULN elevation in ALT and simultaneous elevation of TB exceeding 2 × ULN.
R = [latex]\frac{ALT ÷ ULN of ALT}{Alkaline phosphatase ÷ ULN of alkaline phosphatase}[/latex] - R ≥ 5 = Hepatocellular injury
- R ≤ 2 = cholestasis
- R > 2 and <5 = Mixed pattern of injury
RUCAM (Roussel Uclaf causality assessment method) is an objective means of assessing causality that it is based on a numerical scoring system.
Hy’s law:
- ≥3 × ULN elevation of ALT or AST with >2 × ULN elevation of total bilirubin, without initial findings of cholestasis, indicated by elevated ALP.
- It is an indicator of severity.
- It is associated with a 10% higher risk of mortality.
Clinicopathologic Classifiation of Drug-induced Liver Disease:
Clinicopathologic classifiation of drug-induced liver injury (DILI):
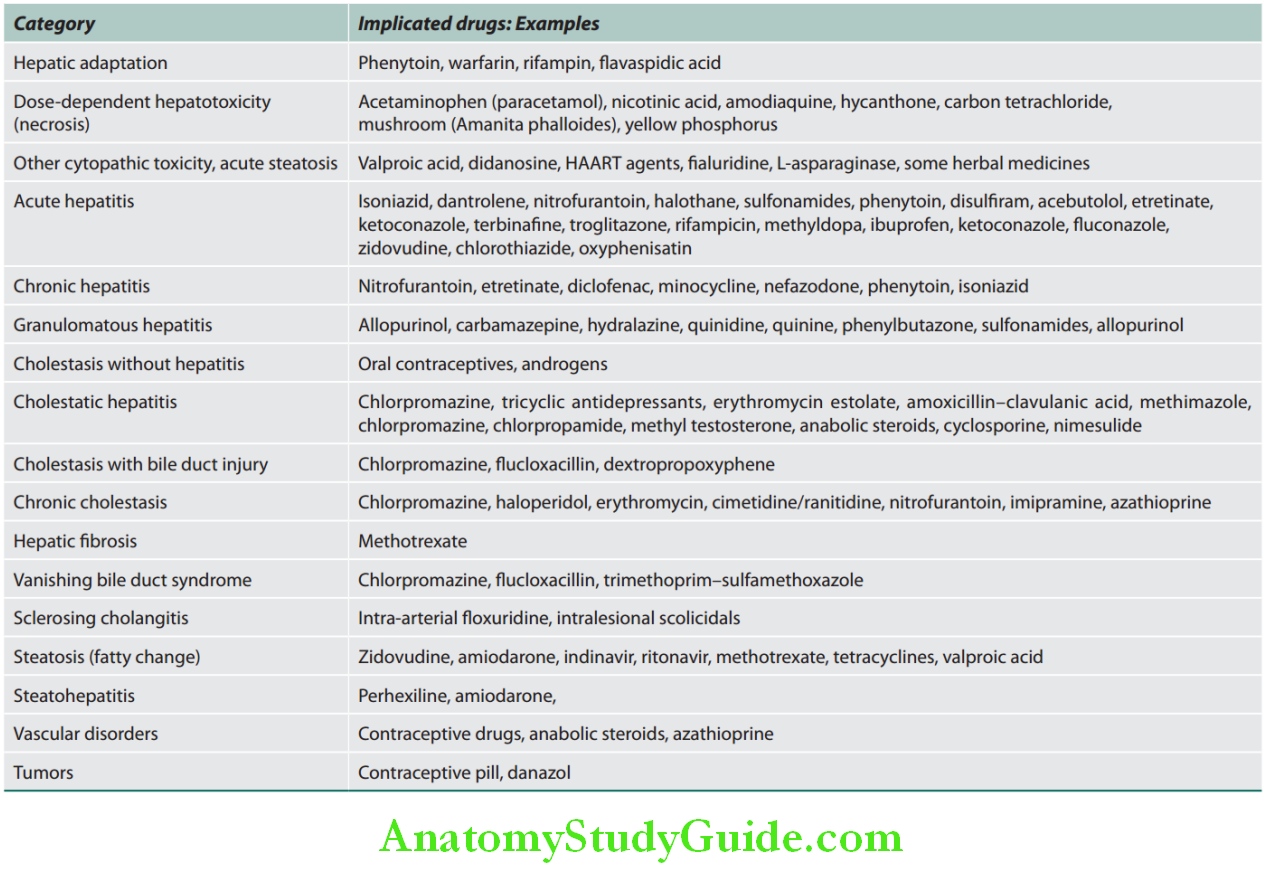
Drug And Toxin-Induced Hepatitis Treatment:
- Discontinue the implicated agent.
- Spontaneous recovery after discontinuation is an important criteria in causality assessment.
- Specific therapy:
- Cholestyramine is used to decrease the course of hepatotoxicity due to specific drugs such as leflunomide and terbinafine. Dose: 4g every 6 hourly for 2 weeks.
- Carnitine is used in valproate toxicity. Dose: 100 mg/kg IV over 30 minutes (maimum dose—6 g), followed by 15 mg/kg every 4 hourly until clinical improvement.
- N-acetyl cysteine is used in paracetamol poisoning.
- Cholestatic DILI can be treated with UDCA at a dose of 13–15 mg/kg
- Corticosteroids have not proven to be beneficial in idiosyncratic DILI unless accompanied by hypersensitivity features such as eosinophilia, rash, and fever.
- Any sign of acute liver failure requires prompt referral to a liver transplant center.
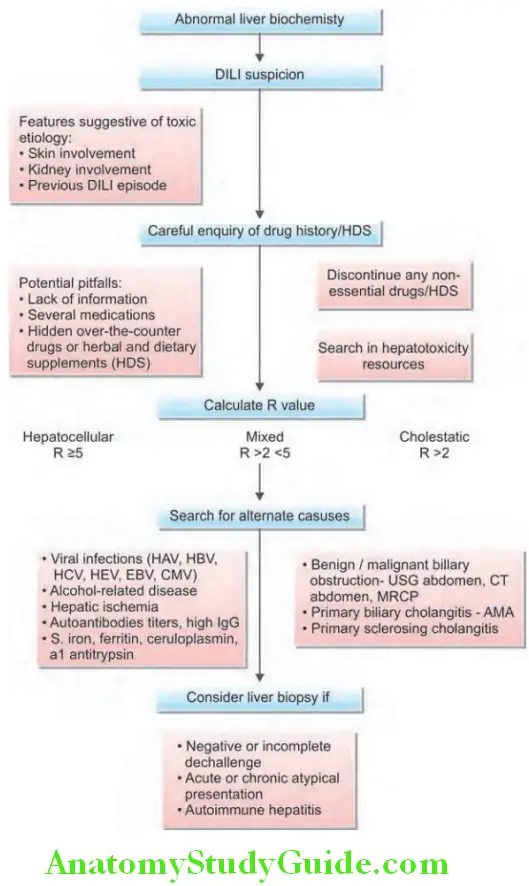
Hepatic Venous Outflow Tract Obstruction:
Question 59. Discuss the etiology, pathology, clinical features, investigations, and management of hepatic venous outflow tract obstruction.
(or)
Describe veno-occlusive disease or sinusoidal obstruction syndrome.
Answer:
- Obstruction to the hepatic venous outflow can occur at different levels. These include:
- Small central hepatic veins: Veno-occlusive disease
- Large hepatic veins: Budd–Chiari syndrome
- Inferior vena cava
- Heart
- Clinical features: It depends on the cause and on the speed with which obstruction develops. Common features are congestive hepatomegaly and ascites.
Budd–Chiari Syndrome:
Question 60. Explain briefly about Budd–Chiari syndrome.
Answer:
- Definition: Budd–Chiari syndrome is characterized by obstruction of hepatic venous outflow owing to occlusion of the hepatic vein.
- Level of obstruction: It may be at any level from the small hepatic veins to the junction of the IVC with the right atrium.
- Classic Budd–Chiari syndrome results from thrombosis of one or more hepatic veins at their openings into the IVC.
Budd–Chiari Syndrome Etiology:
Various causes of Budd–Chiari syndrome:
Hepatic vein obstruction:
Venous thrombosis
- Hypercoagulability states
- Hematological disorders
- Polycythemia vera
- Paroxysmal nocturnal hemoglobinuria
- Antithrombin III, protein C, or protein S deficiencies
- Antiphospholipid syndrome
- Sickle cell disease
- Leukemia
- Pregnancy
- Use of oral contraceptive pills
Compression (may also produce thrombosis):
- Hepatic infections
- Hydatid cyst
- Liver abscess
- Obstruction due to tumors
- Renal cell carcinoma
- Adrenal tumors
- Hepatocellular carcinoma
- Posterior abdominal wall sarcomas
- Radiation injury
- Congenital venous webs
- Trauma to the liver
- Idiopathic (40–50% of cases)
Budd–Chiari Syndrome Clinical Features:
Budd–Chiari syndrome:
It comprises a triad of:
- Abdominal Pain
- Ascites
- Hepatomegaly with hepatic histology showing centrilobular sinusoidal distension and pooling.
- Acute form (acute Budd–Chiari)
- Follows sudden venous occlusion (e.g., by renal cell carcinoma, HCC, and polycythemia)
- Acute upper abdominal pain, nausea, vomiting, tender hepatomegaly, marked ascites, and mild jaundice
- With total venous occlusion: Delirium, coma, and hepatocellular failure
- Fulminant form (fulminant Budd–Chiari syndrome)
- Presents with fulminant hepatic failure usually in the setting of an additional predisposing factor (e.g., factor V Leiden mutation)
- Occurs particularly in pregnant women
- Chronic form (chronic Budd–Chiari)
- More gradual occlusion and more usual presentation
- Pain in abdomen, tender hepatomegaly, and gross ascites
- Enlarged caudate lobe of the liver becomes palpable
- Jaundice is mild or absent
- Splenomegaly with portal hypertension
- Negative hepatojugular reflux, i.e., pressure over the liver fails to fill the jugular veins.
- Bilateral pedal edema and distended veins over abdomen, flanks, and back, with IVC obstruction
- Features of cirrhosis and portal hypertension in patients who survive the acute event
- HCC may develop.
Budd–Chiari Syndrome Investigations:
- Liver function tests vary considerably.
- Features of acute hepatitis: Mild hyperbilirubinemia, raised ALP, low serum albumin, and raised transaminases
- Ascitic fluid examination: Typically shows a high protein content (>5 g/dL, i.e. exudate) in the early stages; however, this often falls later in the disease.
- Ultrasound: It may show enlargement of the caudate lobe, intrahepatic collaterals, echogenic areas, and ascites. It also may show compression of the IVC, if present.
- Pulsed Doppler sonography or a color Doppler: It may show obliteration of the hepatic veins and reversed flow or associated thrombosis in the portal vein with high accuracy. This may be sufficient to establish the diagnosis. Doppler ultrasonography, with sensitivity and specificity rates >80%, is the diagnostic procedure of first choice.
- Noninvasive CT or MRI: It may also demonstrate occlusion of the hepatic veins and IVC with diffuse abnormal parenchyma on contrast-enhancement.
- It may also demonstrate enlargement of the caudate lobe which has independent blood supply and venous drainage.
- Hepatic venography is only necessary if CT and MRI are unable to demonstrate the hepatic venous anatomy clearly. It helps to determine extent of block and caval pressures.
- Liver biopsy: It shows centrilobular congestion hemorrhage, fibrosis, and cirrhosis depending on the duration of the disease.
- Other investigations: To identify a cause (e.g., blood tests and coagulation studies).
Budd–Chiari Syndrome Management:
- Predisposing causes should be removed or treated as far as possible.
- In the acute situation with recent thrombosis: Treated with thrombolytic therapy consisting of intrahepatic venous streptokinase (in very early cases of thrombosis), followed by heparin and oral anticoagulation (warfarin).
- Short hepatic venous strictures: Treated with angioplasty
- Extensive hepatic vein occlusion: Insertion of a covered TIPSS followed by anticoagulation may be useful in opening of the hepatic veins.
- Direct intrahepatic portocaval shunt (DIPS) is a modification of the TIPS procedure, using intravascular ultrasound-guidance, combined with fluoroscopy.
- Ascites: Initially treated medically with low-salt diet, diuretics as well as treating the underlying cause (e.g., polycythemia). If not relieved may be treated with surgical shunts such as Le Veen shunt and portosystemic shunts.
- Percutaneous balloon angioplasty is performed for membranous obstruction of the IVC and hepatic vein.
- Congenital web can be treated radiologically or resected surgically.
- Liver transplantation is indicated for chronic Budd–Chiari syndrome and for progressive liver failure, followed by lifelong anticoagulation.
- Without transplantation or shunting, particularly acute and fulminant types, are associated with poor prognosis.
Veno-occlusive Disease:
Veno-occlusive disease (VOD), also known as sinusoidal obstruction syndrome, is a rare condition characterized by widespread occlusion of the small central hepatic veins.
Budd–Chiari Syndrome Etiology:
- Develops as a complication of:
- Total body irradiation/myeloablative regimens are used before hematopoietic stem cell transplantation. It carries a high mortality.
- A variety of antineoplastic drugs have been implicated as causes of sinusoidal obstruction syndrome including gemtuzumab ozogamicin, actinomycin D, dacarbazine, cytosine arabinoside, mithramycin, and 6-thioguanine.
- Chronic immunosuppression with azathioprine or 6-thioguanine
- Ingestion of herbal teas made with pyrrolizidine alkaloids in Senecio and Heliotropium plants
- Ingestion of alkaloids in inadequately winnowed wheat or in “bush tea”
- Hepatic irradiation
Budd–Chiari Syndrome Clinical Features:
Clinical features are similar to those of the Budd–Chiari syndrome. Classically, sinusoidal obstruction syndrome manifests with mild hyperbilirubinemia (bilirubin levels >2 mg/dL), painful hepatomegaly, weight gain of >2%, and development of ascites.
Budd–Chiari Syndrome Investigations:
- Large hepatic veins appear patent radiologically. This is in contrast to Budd–Chiari syndrome.
- Transjugular liver biopsy (with portal pressure measurements) confirms diagnosis.
- Liver biopsy: Evidence of venous outflow obstruction
Budd–Chiari Syndrome Treatment:
- Supportive and includes control of fluid overload, ascites, and hepatocellular failure.
- Defibrotide is a novel oligodeoxyribonucleotide with anti-ischemic, antithrombotic, and thrombolytic activity but minimal systemic anticoagulant effect. Several studies have shown efficacy of defibrotide in the prevention and treatment of sinusoidal obstruction syndrome with no major toxicity.
Hepatocellular Carcinoma:
Question 61. Discuss the etiology, clinical features, investigations, and management of hepatocellular carcinoma.
Answer:
Hepatocellular carcinoma is the most common primary malignancy of liver from hepatocytes or their precursors.
Predominantly in males with M: F ratio of 4: The number of men and number of women with HCC in the absence of cirrhosis are almost equal.
Hepatocellular Carcinoma Etiology:
Risk factors for hepatocellular carcinoma:
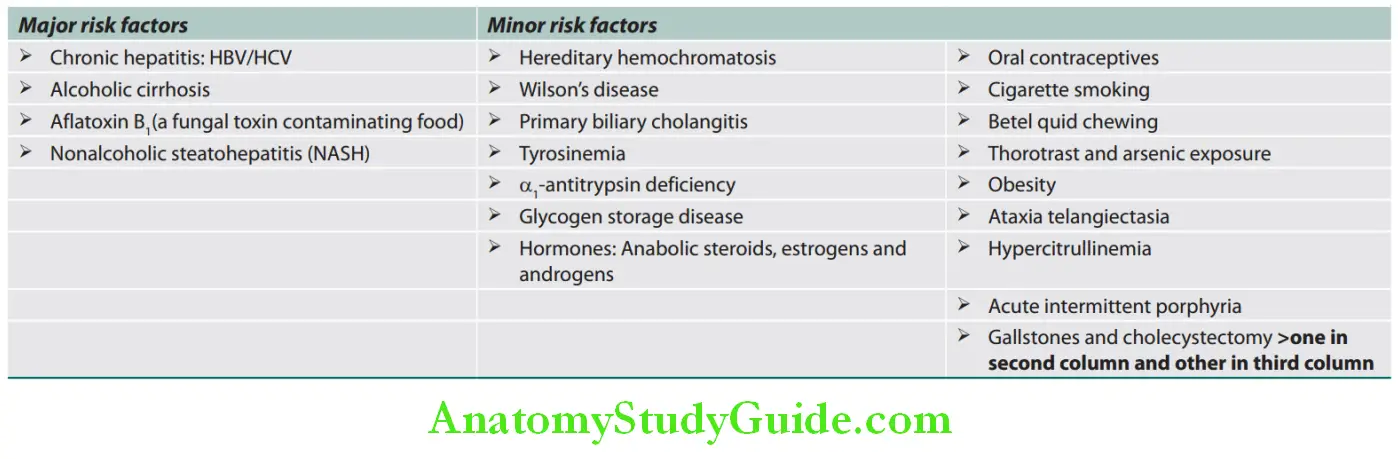
Hepatocellular Carcinoma Clinical Features:
- Usually develops in patients with underlying cirrhosis
- Nonspecific symptoms Include ill-defined upper abdominal pain in the right hypochondrium, malaise, weakness, anorexia, fatigue, weight loss and ascites. Rapid development of these symptoms in a patient with cirrhosis is suggestive of HCC. Fever occurs due to tumor necrosis.
- On examination:
- Liver is enlarged, irregular, and nodular with pain or tenderness.
- Friction rub or a hepatic bruit over the liver due to vascularity of tumor
- Blood-tinged ascites, bone pain, and dyspnea due to metastasis.
Paraneoplastic Syndromes Associated with Hepatocellular:
Carcinoma:
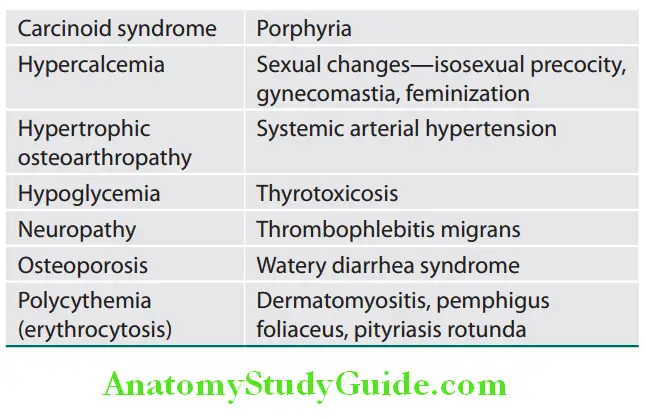
All patterns of HCCs have a strong tendency for invasion of vessels.
The portal vein and its branches are infiltrated by tumor. Occasionally, long, snake-like tumor masses may invade the portal vein and occlude portal circulation. Rarely tumor may invade IVC and extend into the right side of the heart through the hepatic veins. It may metastasize to the lungs.
Hepatocellular Carcinoma Investigations:
- Serum marker
- Alpha-fetoprotein: About 50% HCC is associated with high serum (>500 µg/L) or rising levels of alpha-fetoprotein.
- However, α-fetoprotein levels are often raised in other neoplastic and nonneoplastic liver diseases and in some extrahepatic disorders. AFP concentrations are normal in up to 40% of small HCCs.
- α-l-fucosidase: It is raised in HCC and also in cirrhosis.
- Serum des-γ-carboxy prothrombin: It is raised in a majority of HCC.
- Plasma microRNA expression is also marker of HCC.
- Serum ALP: Very high.
- Ultrasound scans show filling defects.
- CT scan (triple-phase) or MRI abdomen has 75–85% sensitivity in picking HCC. Hyperenhancement on late arterial phase images and washout at the portal venous and/or delayed phases of multiphasic contrast material-enhanced imaging are seen.
- Hepatic artery angiography shows “tumor blushes.”
- Liver scintigraphic scans.
- Liver aspiration or biopsy particularly under ultrasonic guidance confirms the diagnosis.
Hepatocellular Carcinoma Management/Treatment:
Treatment is different for patients with cirrhosis and those without. Therapy depends on tumor size, multicentricity, extent of liver disease (Child–Pugh score) and performance status.
The Barcelona-Clínic Liver Cancer (BCLC) staging system:
- Stage 0 with very early HCC are optimal candidates for resection. Stage A – early HCC are candidates for radical therapies (resection, liver transplantation or percutaneous treatments).
- Stage B- intermediate HCC—chemoembolization. Stage C -advanced HCC -new agents in the setting of clinical trials ,
- Stage D-end-stage disease—symptomatic treatment
- Surgical resection: It is indicated when lesions 1–3 in number and <5 cm in size; without metastasis; with child score A. Local or segmental resections are preferred to major resections. After successful resection, tumor recurs in the cirrhotic liver in about 70% of patients after 5 years.
- Nonsurgical therapy: Majority of patients diagnosed in the advanced stage of HCC and cannot be treated by surgical resection. These patients can be treated by following nonsurgical therapy.
- Local ablation strategies:
- Radiofrequency ablation (RFA): Percutaneous RFA uses heat to kill tumor cells. A single electrode inserted into the tumor under CT or ultrasound guidance.
- Transarterial embolization (TAE): Hepatic artery embolization with Gelfoam and doxorubicin
- Transarterial chemo-embolization (TACE): With drugs such as doxorubicin but is contraindicated in decompensated cirrhosis and when HCC is multifocal can be combined with RFA.
- Local injection therapy: Numerous agents can be used for local injection into tumors and includes percutaneous ethanol injection (PEI) or percutaneous acetic acid injection (PAI). PEI causes not only direct destruction of tumor cells, but also destroy normal cells in the vicinity. It usually requires multiple injections (average three) and the maximum size of tumor treated is 3 cm.
- Conventional chemotherapy and radiotherapy are unsuccessful.
- Chemotherapy using sorafenib: This drug is a multikinase inhibitor with activity against RAF, VEGF, and PDGF signaling.
- Nivolumab against programmed cell death 1 receptor (PD-1) has been used as immunotherapy.
- Local ablation strategies:
- Liver transplantation: Indicated in presence of localized tumor and underlying advanced liver disease. Unfortunately, the underlying liver disease (e.g., hepatitis B and C) may recur in the transplanted liver.
Hepatocellular Carcinoma Prognosis:
Depends on size of tumor, the extent of spread (e.g., presence of vascular invasion), and liver function in those with cirrhosis.
Liver Transplantation:
Question 62. Discuss briefly about liver transplantation.
Answer:
- Transplantation of liver is useful in treating patient with end-stage liver disease or acute/fulminant liver failure and 5-year survival rate in good centers is about 75%.
- “Split” livers: In which one liver is used for two recipients. It is helpful in tackling organ shortage and in shortening the
time on the waiting list. - Orthotopic liver transplantation: It is the most common form of liver transplantation. Orthotopic means that the graft is placed in its correct anatomical location. In this technique, donor organ after removal of the native organ is transplanted in the same anatomic location.
- Auxiliary partial orthotopic liver transplantation (APOLT) is also called partial or split liver transplantation.
- In this technique, a segment of donor liver is transplanted in a recipient who has undergone hemihepatectomy to make room for the graft.
- Advantage:
- If the donor’s liver fails, the recipient’s own organ can function as a backup until a new liver is found.
- Living donor liver transplantation: In this technique, a portion of healthy person’s liver is removed and used for transplantation.
- Living-related donors: In this, the donor of the liver segment is a first-degree living relative.
- Bioartificial liver: Cultured hepatocytes are used in patients with acute liver failure till donor liver becomes available.
Liver Transplantation Indications:
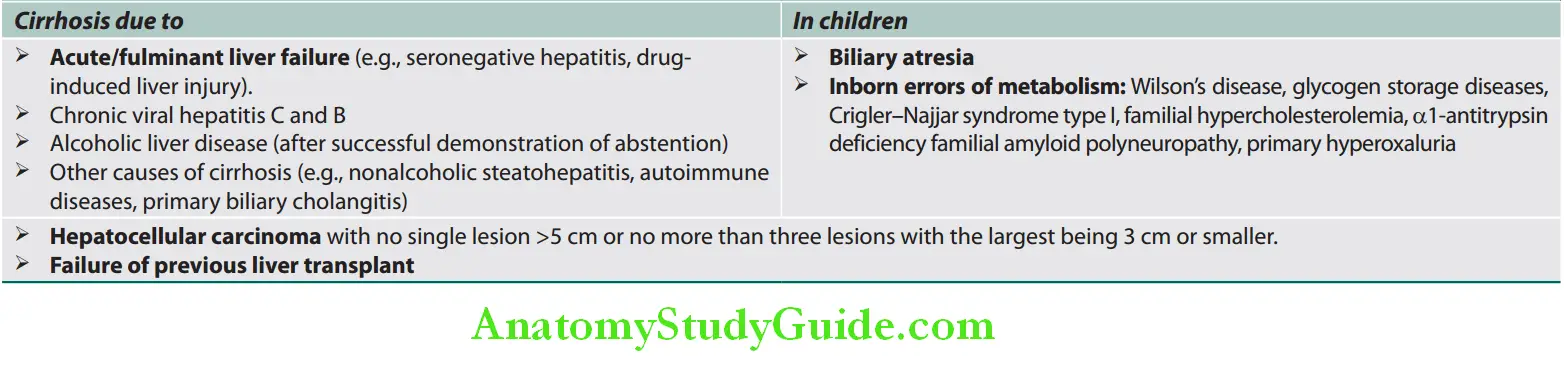
Contraindications:
- Absolute contraindications:
- Poor expected outcome of transplantation (e.g., multisystem organ failure, malignancy or infection of extrahepatic or extrabiliary tract, HCC with metastatic spread advanced cardiac or pulmonary disease, and HIV infection).
- Relative contraindications:
- Comorbidities that reduce survival (e.g., renal insufficiency, primary hepatobiliary malignancy >5 cm, hemochromatosis, SBP, patient older than 65 years).
Complications of Liver Transplantation and Immunosuppression:
Complications of immunosuppression:
- Infections
- Metabolic syndrome
- Hypertension
- Diabetes mellitus
- Obesity
- Dyslipidemia
- Cardiovascular risk
- Acute and chronic renal disease
- Metabolic bone disease
- De novo malignancy
Complications of liver transplantation:
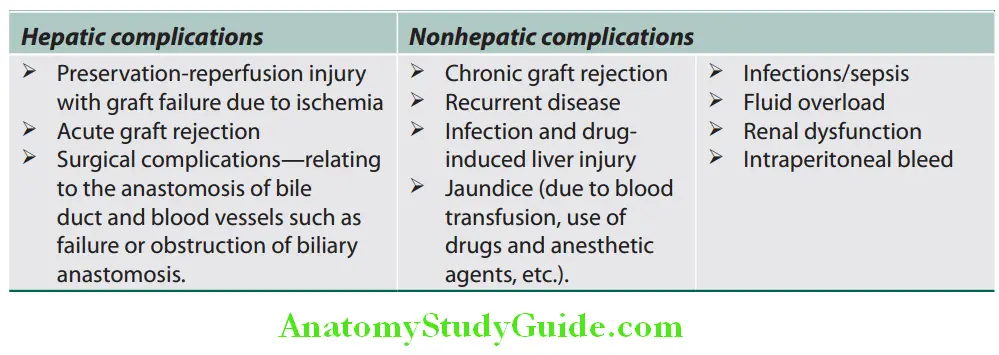
Pyogenic Abscess:
Question 63. Discuss the etiology, clinical features, investigations, and management of pyogenic liver abscess (bacterial liver abscess).
Answer:
Pyogenic Abscess Etiology:
- Bacterial infections in the liver may be manifested as pyogenic abscess. The most common organism includes E.colStreptococcus milleri, Klebsiella pneumoniae, and Bacteroides. Other organisms include Enterococcus faecalis, Proteus vulgaris, and Staphylococcus aureus. Often the infection is mixed. Tuberculosis and melioidosis can present with liver abscess.
- Route of infection: The organisms may reach the liver through one of the following routes:
- Portal vein: Major source is intra-abdominal infections (e.g., appendicitis, diverticulitis, colitis and perforated bowel).
- Arterial blood supply: During systemic bacteremia, organism may reach liver via hepatic artery.
- Ascending infection in the biliary tract (ascending cholangitis).
- Direct invasion of the liver from a nearby source (e.g., subphrenic abscess, perinephric abscess), or a penetrating injury.
Pyogenic Abscess Clinical Features:
- Fever, chills, rigors, and right upper quadrant pain radiating to right shoulder
- Weight loss, anorexia, nausea, and vomiting; Can manifest as PUO
- Pleuritic chest pain
- Tender hepatomegaly
- Mild jaundice may develop when there is extrahepatic biliary obstruction.
- Respiratory findings at the base of right lung (pleural effusion and crepitations) or a pleural rub in the right lower chest.
Pyogenic Abscess Investigations:
- Serum bilirubin: Raised in 25% of cases
- Serum ALP: Markedly elevated
- Blood cultures: Positive in only 30% of cases.
- Normochromic normocytic anemia, usually accompanied by a polymorphonuclear leukocytosis
- ESR and CRP are often raised.
- Chest radiograph: Elevation of the right dome of diaphragm, and in severe cases right basilar atelectasis and pneumonia or effusion
- Ultrasonography confirms the diagnosis.
- CT scan of abdomen: helpful when ultrasound is normal.
- Needle aspiration of pus for culture and sensitivity
Pyogenic Abscess Management:
- Antibiotics: Initiate treatment with antibiotics (combination of ampicillin, gentamicin, and metronidazole) to cover gram-positive, gram-negative, and anaerobic organisms till the causative organism is identified. Later, change the antibiotic according to the culture and sensitivity reports. Duration of treatment is 4–8 weeks.
- Ultrasound-guided aspiration of the abscess: Indications are listed.
- Surgical drainage via a large-bore needle for those who fail to respond.
- Treat the underlying cause
Indications for ultrasound-guided aspiration of liver abscess:
- Large abscess (>6 cm).
- Abscess in the left lobe.
- Lack of response within 48-72 hours of medical therapy
- Ultrasonography suggestive of large abscess impending rupture
Amebic Liver Abscess:
Question 64. Write short essay/note on diagnosis and management of amebic liver abscess/hepatic amebiasis.
Answer:
Liver involvement by Entamoeba histolytica produces amebic liver abscess. The term amebic hepatitis is not used at present.
Amebic Liver Abscess Pathogenesis:
- Entamoeba histolytica trophozoites from the base of an amebic ulcer in colon may reach liver through the portal circulation. The capillary system of the liver acts as an efficient filter and holds these trophozoites. Trophozoites multiply, kill hepatocytes, cause coagulation necrosis of the liver cells and produce single or multiple abscesses.
- Amebic abscess ranges from 8–12 cm in diameter and appears well circumscribed. In most of the cases, the abscess is single and confined to the posterosuperior aspect of the right lobe of the liver. This is because right-lobe portal laminar blood flow is supplied predominantly by the superior mesenteric vein, whereas the leftlobe portal blood flow is supplied by the splenic vein. Abscess cavity is filled with a thick, dark brown, odorless, semisolid necrotic material, which resembles anchovy paste (sauce) in color and consistency.
Amebic Liver Abscess Clinical Features:
- Symptoms are similar to pyogenic abscesses (such as fever, anorexia, weight loss and malaise). Concurrent diarrhea is present in less than one third of patients.
- Insidious/gradual onset with low-grade fever, sweats, malaise, weight loss, chills, and rigors. Few may have an acute-onset of fever. In later phase, there may be swinging temperature and sweating usually without marked systemic symptoms or associated cardiovascular signs.
- Pain: Patient may have pain in the right hypochondrium due to stretching of the liver capsule. Diaphragmatic irritation by abscess may cause referred pain in the right shoulder.
- Past history of dysentery may be present. Jaundice is rare.
- Physical examination: The patient looks ill, shows an enlarged, tender liver, intercostals point tenderness in the posterolateral of a lower right intercostals space (intercostal tenderness) and bulging of the intercostals spaces, upward extension of the liver dullness on percussion. There may be signs of an effusion or consolidation in the base of the right side of the chest.
Amebic Liver Abscess Complications:
- Due to rupture of abscess:
- Spontaneous external rupture may produce “granuloma cutis.”
- Rupture into bronchus may result in expectorates large amounts of the typical “anchovy-sauce”pus
- Rupture into pleural space may produce massive pleural effusion
- Rupture into peritoneal cavity may produce peritonitis
- Rupture below the diaphragm to produce subdiaphargmatic abscess
- Rupture into stomach
- Abscess in the left lobe of liver may rupture into pericardium resulting in pericarditis and rarely cardiac tamponade.
- Due to direct extension into lung, it may produce amebic lung abscess.
- Due to hematogenous spread: Metastatic brain abscess, splenic abscess
Amebic Liver Abscess Diagnosis:
- Blood: Polymorphonuclear leukocytosis is a characteristic finding.
- Liver function tests: May be abnormal.
- Most consistent abnormality is raised ALP.
- Level of AST reflects the severity of the disease. Jaundice is uncommon, and its presence is indicative of a grave prognosis.
- Chest radiography: Demonstrate abscess (a raised right hemidiaphragm on chest X-ray). Other findings include rightsided pleural effusion and right basal pneumonitis.
- Ultrasound: Ultrasonic scanning of liver is very useful for establishing the diagnosis and localization of the abscess. The defect produced by abscess in the liver usually persists for several months after the complete recovery of the patient.
- Isotope liver scans and computed tomography: Can also assist to detect abscess
- Serologic tests: For ameba, include indirect hemagglutination test, amebic complement fiation test, ELISA or counter immunoelectrophoresis which can detect antibodies in the blood and are more useful in amebic liver abscess.
- Amebic fluorescent antibody test: It is positive in about 90% of patients with liver abscess and in 60–70% with active colitis. Nested-multiplex PCR has a sensitivity of 75–100%.
- Aspiration of amebic liver abscess: Needle aspiration yields the characteristic “anchovy-sauce” or “chocolate-brown” pus. The pus is usually thick in consistency, yellow or green in color, and characteristically odorless. It consists of liquefied necrotic liver tissue and does not contain polymorphonuclear leukocytes. As the parasites are localized in the abscess wall, pus may not show free amebae. Ameba may be demonstrated in the terminal portion of the aspirate, or by a needle biopsy of the abscess wall.

Amebic Liver Abscess Treatment:
- Metronidazole 800 mg three times daily for 7–10 days or tinidazole 2 g orally daily as a single dose for 5 days is usually adequate in liver abscess. Severe cases may need intravenous administration of metronidazole (500 mg 8 hourly).
- Chloroquine: It may be given at a dose of 300 mg twice daily for 2 days, followed by 150 mg twice daily for another 19 days. It is usually administered to those patients who do not respond adequately to metronidazole.
- Emetine and dehydroemetine are lethal to the trophozoites of E. histolytica. However, because of their toxicity, they are rarely used.
- Dehydroemetine is less toxic than emetine. Toxicity includes cardiac arrhythmias, muscle weakness, vomiting precordial pain, and diarrhea.
- Therapeutic aspiration of the abscess: If a liver abscess is large or likely to burst, or if the response to chemotherapy is not prompt, aspiration is required and repeated if required. Rupture of an abscess into the pleural cavity, pericardial sac or peritoneal cavity needs immediate aspiration or surgical drainage.
- Percutaneous drainage: It is achieved by placing a large-bore catheter into the abscesses for draining the abscess.
- It is usually performed when therapeutic aspiration fails and its indications are:
- Large abscess and associated risk of spontaneous rupture (especially left-lobe abscesses)
- If abscess is ruptured already (drainage of both abscess and extraneous collection)
- Absence of response to medical therapy with signs of persistent sepsis or enlarging abscesses or persistent symptoms
- Evidence of liver failure
- After treatment of the invasive disease, luminal amebicide such as diloxanide furoate 500 mg orally TID for 10 days; or aminosidine (paromomycin) 25–35 mg/kg/day orally in three divided doses for 7–10 days should be given to clear luminal cysts or parasite. Alternative agents include iodoquinol 650 mg orally TID for 20 days, and nitazoxanide.
Clinical comparison between pyogenic and amebic liver abscess:
Pyogenic and amebic liver abscess: clinical comparisons:
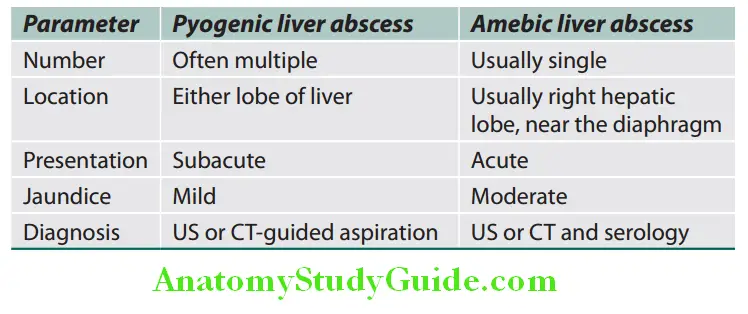
Metabolic Liver Disease:
Iron Overload:
Question 65. What are various disorders associated with iron overload?
(or)
Discuss the etiology, pathology, clinical features, investigations, and management of hereditary (primary) hemochromatosis (“bronze diabetes”).
Answer:
Classifiation of Iron Overload:
In secondary iron overload, iron accumulates in Kupffer cells rather than hepatocytes compared to that of hereditary hemochromatosis.
Classification of iron overload:
- Hereditary:
- Mutations of genes encoding HFE, transferrin receptor 2 (TfR2), or hepcidin
- Mutations of genes encoding hemojuvelin (HJV)
- Hemosiderosis (secondary hemochromatosis):
- Parenteral iron overload
- Exogenous: Multiple blood transfusions, repeated iron
injections, long-term hemodialysis - Endogenous: Sickle cell disease
- Exogenous: Multiple blood transfusions, repeated iron
- Ineffective erythropoiesis with increased erythroid activity: β-thalassemia, sideroblastic anemia, porphyria cutanea tarda
- Increased oral intake of iron
- Chronic liver disease: Chronic alcoholic liver disease
Hereditary Hemochromatosis:
- Hereditary hemochromatosis (HH) is an inherited autosomal recessive disorder characterized by abnormal (excessive) accumulation of iron in various parenchymal organs leading to eventual fibrosis and functional organ failure.
- In symptomatic patients of hemochromatosis, the total body iron is increased to 20–40 g, compared with 3–4 g in normal individuals.
- Associated with high incidence of HCC:
- Most of the hereditary hemochromatosis is inherited as autosomal recessive genetic disorder and associated with HLA-B3, B7 and B14 histocompatibility antigens.
Metabolic Liver Disease Pathology:
The excess iron is deposited commonly in the liver, joint, heart, pancreas, endocrine glands (e.g., pituitary gland) and skin.
Metabolic Liver Disease Clinical Features:
- Age and gender: Overt clinical manifestations occur more frequently in males over the age of 40 years. About 90% of the patients are males. Females are protected because of loss of iron during menstruation and pregnancy.
- Symptoms:
- It may develop due to toxic damage of cells by accumulated iron and consequent fibrosis.
- Symptoms may be vague-muscle aches, weakness, abdominal and/or joint pain.
- Classic triad: Consists of bronze skin pigmentation (due to melanin deposition in exposed parts, axillae, groins and genitalia), hepatomegaly and diabetes mellitus (“bronzed diabetes”) is observed in patients with gross iron overload.
- Late features: Hypopituitarism, loss of libido, testicular atrophy, cardiac complaints (cardiomyopathy, heart failure and cardiac arrhythmias), hypothyroidism, cirrhosis with hepatosplenomegaly, spiders, loss of body hair, jaundice, and ascites.
- Cirrhosis
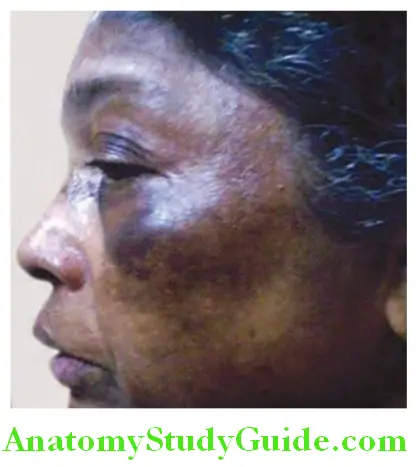
Metabolic Liver Disease Complications:
- Chondrocalcinosis: Develops due to asymmetrical deposition of calcium pyrophosphate in both large and small joints and leads to an arthropathy. It has characteristic radiologic findings: Squared-off bone ends and hook-like osteophytes in the second and third metacarpophalangeal (MCP) joints.
- Hepatocellular carcinoma in 30% of patients with cirrhosis
- Multi-organ failure
- Susceptibility to specific infections (bacteria whose virulence is increased in the presence of excess iron). These include Listeria monocytogenes, Yersinia enterocolitica, and Vibrio vulnificus.
Metabolic Liver Disease Investigations:
- Serum iron profile:
- Serum iron is elevated (>30 μmol/L).
- Total iron binding capacity (TIBC): Reduced
- Fasting transferrin saturation (serum iron divided by the total iron binding capacity) is high (>45%) which is highly sensitive for diagnosis.
- Serum ferritin is elevated (usually >500 μg/L or 240 nmol/L). It is less sensitive than transferrin saturation in screening for hemochromatosis because it is also increased in alcoholic liver disease, hepatitis C infection, NASH. It is also a acute-phase reactant and increased in other inflammatory and neoplastic conditions.
- Biochemical tests for liver function are often normal, even with established cirrhosis.
- Genetic testing: It is performed if iron studies are abnormal.
- CT scan: Shows increased density of liver due to deposits of iron.
- Magnetic resonance imaging (MRI): It is sensitive to detect liver iron content.
- Liver biopsy: Not necessary for diagnosis. It shows iron deposition and hepatic fibrosis leading on to cirrhosis.
- Screening: All first-degree family members of hereditary hemochromatosis must be screened to detect early and asymptomatic disease.
Metabolic Liver Disease Treatment and Management:
- Treatment should be started before permanent organ damage occurs due to iron toxicity. The excess iron should be removed as rapidly as possible and prolongs life and may reverse tissue damage.
- Phlebotomy/Venesection: Venesection of 500 mL blood (removes 250 mg of iron) is performed twice-weekly until the serum iron is normal. This may take 2 years or more.
- Chelation therapy: Rarely in patients who cannot tolerate venesection because of severe cardiac disease or anemia, chelation therapy with desferrioxamine can be used. Dose is 40–80 mg/kg/day subcutaneously. It removes about 10–20 mg of iron/day. Deferiprone and deferasirox are other chelators.
- Erythrocytapheresis is an apheresis technique whereby red cells are removed in an isovolemic manner and the patient’s plasma is returned to the patient, larger amounts of iron can be removed per session than by phlebotomy.
- Treatment of diabetes
- Treatment of cirrhosis: There is a risk of malignancy if cirrhosis is present
- Treatment of congestive heart failure and cardiac arrhythmias
- Dietary limitations:
- Dietary iron intake to be restricted
- Agents for reducing iron absorption (tannates in tea, phytates, oxalates, calcium, and phosphates) to be used,
- Avoidance Of Excessive Ethanol,
- Avoidance Of Ascorbic Acid (Vitamin C) Supplements
- Avoidance of uncooked seafood.
Wilson’s Disease:
Question 66. Discuss the etiology, pathology, clinical features, investigations, and management of Wilson’s disease (hepatolenticular degeneration).
(or)
Explain in brief about Kayser-Fleischer ring.
Answer:
- Wilson’s disease (progressive hepatolenticular degeneration) is a very rare inborn error of copper metabolism characterized by increased total body copper.
- Excess copper deposition in various organs:
- Liver
- Basal Ganglia Of The Brain,
- Cornea,
- Kidneys
- Skeleton.
- Potentially treatable condition.
Wilson’s Disease Etiology:
- Autosomal recessive disorder
- Consanguinity is risk factor.
- Molecular defect within a copper-transporting ATPase encoded by a gene (ATP7B) located on chromosome 1More than 300 mutations have been identified and is rare in India and Asia.
- Defect: Excessive accumulation of copper in the body due to:
- Failure of incorporation of copper into proceruloplasmin and leads to low serum ceruloplasmin.
- Failure of biliary copper excretion, causing its accumulation in the body.
Wilson’s Disease Pathology:
Liver: Microscopic features are not diagnostic and vary from that of chronic hepatitis to macronodular cirrhosis. Stains for copper show a periportal distribution of copper.
Wilson’s Disease Clinical Features:
- Age of presentation is usually between 5 and 30 years.
- Children usually present with hepatic problems.
- Young adults usually present with more neurological problems.
- Features of liver involvement: Varies from
- Acute Hepatitis Going On To
- Fulminant Hepatic Failure, To
- Chronic hepatitis, or
- Cirrhosis
- Features of brain involvement: Dysarthria, involuntary movements, tremors (especially asymmetric, resting and intention tremors, wing beating), ataxia, and eventually dementia.
- Psychiatric manifestations: Phobia, depression, compulsive behavior
- Kayser–Fleischer rings:
- It is a characteristic sign due to deposition of copper in the Descemet’s membrane of cornea.
- Appears as greenish-brown or golden-brown ring at the corneoscleral junction (around the periphery of the cornea), appearing first at the upper periphery gradually disappear with effective medical treatment or liver transplantation.
- Best identified by slit-lamp examination
- I t may be absent in young children and disappears with treatment.
- May be associated with “sunflower cataracts.”
- Kayser–Fleischer rings are not specific for Wilson’s disease; they are found occasionally in patients with other types of chronic liver disease, usually with a prominent cholestatic component, such as PBC, primary sclerosing cholangitis, or familial cholestatic syndromes, and rarely in patients with nonhepatic diseases.
Other manifestations: Kidney (renal tubular damage), skeleton (osteoporosis, arthropathy).
Wilson’s Disease Investigations:
- Slit-lamp examination of the eyes for Kayser–Fleischer ring
- Serum copper: Reduced but can be normal.
- Serum ceruloplasmin levels: Low and less than 20 mg/dL.
- Urinary copper: Usually increased 100–1,000 μg in 24 h (6–16 μmol); normal levels <40 μg (0.6 μmol). The penicillamine challenge: 500 mg dose of penicillamine at the beginning of the 24-hour urine collection and then again at 12 hours.
- Urinary copper excretion greater than 1,600 mg per 24 hours (>25 μmol) is diagnostic.
- Liver biopsy: Gold standard. Diagnosis depends on the amount of copper in the liver (>250 μg/g dry weight).
- Hemolysis and anemia may be found.
Wilson’s Disease Treatment and Management:
- Treatment should be started early and will show improvement both clinically and biochemically.
- Chelating drugs
- Penicillamine with pyridoxine: It should be given lifetime in the dose of 1–5 g daily and effectively chelates copper.
- For asymptomatic cases and maintenance therapy (after maximal improvement with penicillamine)
- Trientine dihydrochloride: In the dose of 2–8 g/day
- Zinc acetate: Indicated in chronic hepatitis and cirrhosis. Dose 150 mg/day. It blocks absorption of copper from intestine. However, it should not be administered with penicillamine or trientine since both these drugs chelate zinc.
- Other drugs:
- Intramuscular Dimercaprol
- Ammonium Tetrathiomolybdate
- Potassium Sulfide
- Carbacrylamine resins.
- Liver transplantation: In fulminant hepatic failure and decompensated/advanced cirrhosis
- All siblings and children of the patient should be screened for Wilson’s disease and treated even if they are asymptomatic, and if there is evidence of copper accumulation.
α1-Antitrypsin Defiiency:
Question 67. Write a short note on α1-antitrypsin.
Answer:
α1-Antitrypsin Defiiency Clinical Features:
- In neonates, it produces cholestatic jaundice
- In adults:
- Liver: Chronic hepatitis, cirrhosis, HCC, and cholangiocarcinoma
- Lung: Emphysema, chronic bronchitis
- Others: Panniculitis, vasculitis, pancreatitis, and glomerulonephritis
α1-Antitrypsin Defiiency Investigations:
- Serum α1-antritrypsin is low (normal above 150 mg/dL).
- Serum protein electrophoresis shows absence of∝1-globulin peak.
- Liver biopsy: α1-AT accumulates in periportal hepatocytes and can be seen as periodic acid-Schiff (PAS)-positive, diastase-resistant globular inclusions in these hepatocytes. The injury to these hepatocytes results in progressive fibrosis and cirrhosis.
α1-Antitrypsin Defiiency Treatment:
- No treatment apart from dealing with the complications of liver disease.
- Stop cigarette smoking and alcohol intake.
- Liver or lung transplantation.
Biliary Cirrhosis:
Question 68. What is biliary cirrhosis? Discuss the etiology, pathology, clinical features, investigations, and management of primary biliary cholangitis.
Answer:
It is a type of cirrhosis of the liver secondary to prolonged obstruction of biliary system (anywhere between the small interlobular bile ducts and the papilla of Vater).
Obstruction results in progressive destruction of bile ducts.
Biliary Cirrhosis Classifiation:
Biliary cirrhosis may be subdivided into PBC and secondary biliary cirrhosis.
- Primary biliary cholangitis: It is a probably an autoimmune disorder of the intrahepatic biliary tree.
- Secondary biliary cirrhosis: It is due to prolonged obstruction of the extrahepatic biliary tree.
Primary Biliary Cirrhosis Presently called as Primary Biliary Cholangitis:
Gender: It usually affects middle-aged women, with a female to male ratio of more than 6
Age: Peak incidence between 40 and 50 years of age
(perimenopausal).
Biliary Cirrhosis Etiology and Pathogenesis:
- PBC is thought to be an autoimmune disorder, but its exact pathogenesis is not known.
- Genetic and environmental factors play role in the pathogenesis of the PBC. Probably an environmental factor acts on a genetically predisposed individual via molecular mimicry initiate autoimmunity.
Biliary Cirrhosis Clinical Features:
- It is insidious in onset and asymptomatic patients are discovered during routine examination or investigations.
- Commonly present with pruritus (often the earliest symptom), fatigue, and skin pigmentation (due to melanin deposition) abdominal discomfort.
- Features of liver involvement:
- Intense pruritus (probably due to bile salts) occurs months to years before jaundice and is more at night. Scratch marks.
- Progressive jaundice (bottle green color)
- Clubbing of fingers
- Hepatosplenomegaly
- Hepatic decompensation, portal hypertension, ascites and variceal bleeding develop.
- Hypercholesterolemia:
- Xanthelasmas (cholesterol-rich macrophages) around the eyes
- Xanthomas over joints, tendons, hand creases, elbows, and knees
- Pain, tingling, and numbness over feet and hands due to peripheral neuropathy produced by lipid infiltration of peripheral nerves.
- Malabsorption
- Steatorrhea and diarrhea due to malabsorption of fat
- Easy bruising and ecchymosis due to vitamin K deficiency
- Osteomalacia and/or osteoporosis due to malabsorption of vitamin D
- Night blindness due to vitamin A deficiency
- Dermatitis due to vitamin E deficiency
- Extrahepatic manifestations: These include autoimmune disorders such as Sjögren syndrome, keratoconjunctivitis sicca (dry eyes and mouth), systemic sclerosis, autoimmune thyroiditis, rheumatoid arthritis, Raynaud phenomenon, membranous glomerulonephritis, and celiac disease.
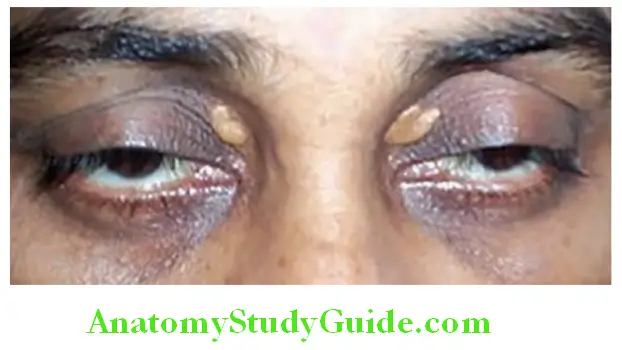
Biliary Cirrhosis Investigations:
- Antimitochondrial antibodies are characteristic and are essential for the diagnosis of PBC. They are found in 90–95% of patients. M2 antibody is specific. Other nonspecific antibodies (e.g., antinuclear factor and smooth muscle) may also be present.
- Marked rise of serum 5’-nucleotidase activity
- High serum ALP (markers of cholestasis): Two-to five-fold rise
- Hyperlipidemia and serum cholesterol is raised.
- Serum bilirubin: Hyperbilirubinemia of the conjugated type occurs in late stages.
- Serum transaminase: Mild elevation of transaminases.
- Serum IgM may be very high.
- Ultrasound: It shows diffuse alteration in liver architecture.
- MRCP (or ERCP): It shows normal biliary tree.
- Liver biopsy: Portal tract infiltration by lymphocytes and plasma cells and about 40% have granulomas. Later portal tract fibrosis and, eventually progress to cirrhosis.
Biliary Cirrhosis Management/Treatment:
- Ursodeoxycholic acid (10–15 mg/kg): It improves bilirubin and aminotransferase levels. It should be started early in the asymptomatic phase.
- Steroids: They improve biochemical and histological disease but may lead to increased osteoporosis.
- Other therapies include azathioprine, colchicines, and methotrexate and cyclosporine may be beneficial in few patients.
- Steatorrhea: It is treated by limiting intake of fat and substituting long-chain triglycerides with medium-chain triglycerides in the diet
- Malabsorption of fat-soluble vitamins (A, D, and K) is managed by supplementation.
- Calcium supplementation
- Pruritus is difficult to control but following can be helpful.
- Cholestyramine one 4 g sachet three times daily, but is unpalatable
- Antihistamines
- Rifampicin, naloxone hydrochloride, ondansetron, and opiate antagonists (naloxone and naltrexone)
- Liver transplantation
Biliary Cirrhosis Complications: These include
- Cirrhosis,
- Osteoporosis And Osteomalacia
- Polyneuropathy
- Increased risk of HCCs.
Secondary Biliary Cirrhosis:
Question 69. Write short note on secondary biliary cirrhosis.
Answer:
Secondary Biliary Cirrhosis Defiition:
Cirrhosis developing secondary to prolonged (for months) obstruction of the extrahepatic (large biliary duct) biliary tree
Causes of Obstruction:
- Gallstones
- Bile Duct Strictures
- Sclerosing cholangitis
Patients with malignant tumors of bile duct or pancreas rarely survive long enough to develop secondary biliary cirrhosis.
Cardiac Cirrhosis:
Question 70. Write short note on cardiac cirrhosis.
Answer:
- Cause: Liver damage primarily due to congestion may develop in all types ofright heart failure. Examples: valvular heart disease, constrictive pericarditis, or cor pulmonale of long duration (more than 10 years).
- Mechanism: Right heart failure causes retrograde transmission of raised venous pressure via the IVC and hepatic vein into the liver. This leads to passive congestion of liver. When passive venous congestion becomes chronic/prolonged, the liver becomes enlarged, tender and shows a “nutmeg appearance” (alternating red-congested and pale-fibrotic areas).
- Very rarely, prolonged, severe right-sided heart failure and hepatic congestion cause cardiac cirrhosis.
- Clinical features:
- Usually dominated by the heart disease
- Symptoms and signs of severe right heart failure.
- Diagnosis:
- Firm enlarged liver with signs of chronic liver disease in a patient with valvular heart disease, constrictive pericarditis, or chronic cor pulmonale
- Nonpulsatile liver despite the presence of tricuspid regurgitation
- Liver biopsy confirms the diagnosis (not required in most cases).
Treatment of the underlying cardiovascular disorder.
Noncirrhotic Portal Fibrosis:
Question 71. Write a short note on noncirrhotic portal fibrosis.
Answer:
- Noncirrhotic portal fibrosis (NCPF) is an idiopathic disease characterized by periportal fibrosis and involvement of small and medium branches of portal vein.
- It leads to development of portal hypertension and splenomegaly without features of liver cell failure.
- Worldwide it accounts for 3–5% of all patients with portal hypertension, but in India it accounts for 15–20% of cases of portal hypertension.
Noncirrhotic Portal Fibrosis Etiology:
Bacterial infections, exposure to toxins (arsenic, vinyl chloride monomer, copper sulfate, methotrexate, hypervitaminosis A, 6-mercaptopurine), immunological abnormalities and hypercoagulable states may play a role.
Noncirrhotic Portal Fibrosis Clinical Features:
- Characterized by upper GI bleed, massive splenomegaly with anemia
- Preserved liver function
- Ascites, jaundice, and hepatic encephalopathy are uncommon.
Noncirrhotic Portal Fibrosis Investigations:
- Liver function tests: Usually normal
- Peripheral blood: Pancytopenia may develop due to hypersplenism.
- Doppler ultrasound: It shows patent splenoportal axis and hepatic veins.
- Splenoportovenography (SPV): It reveals massive dilatation of the portal and splenic veins, and the presence of collaterals.
- Liver biopsy: Lobular architecture maintained, portal fibrosis of variable degree, sclerosis and obliteration of small-sized portal vein radicals.
Noncirrhotic Portal Fibrosis Treatment: Endoscopic sclerotherapy or banding to prevent variceal bleed and shunt surgery.
Differences between extrahepatic portal venous obstruction (EHPVO), NCPF, and cirrhosis are presented in Table:
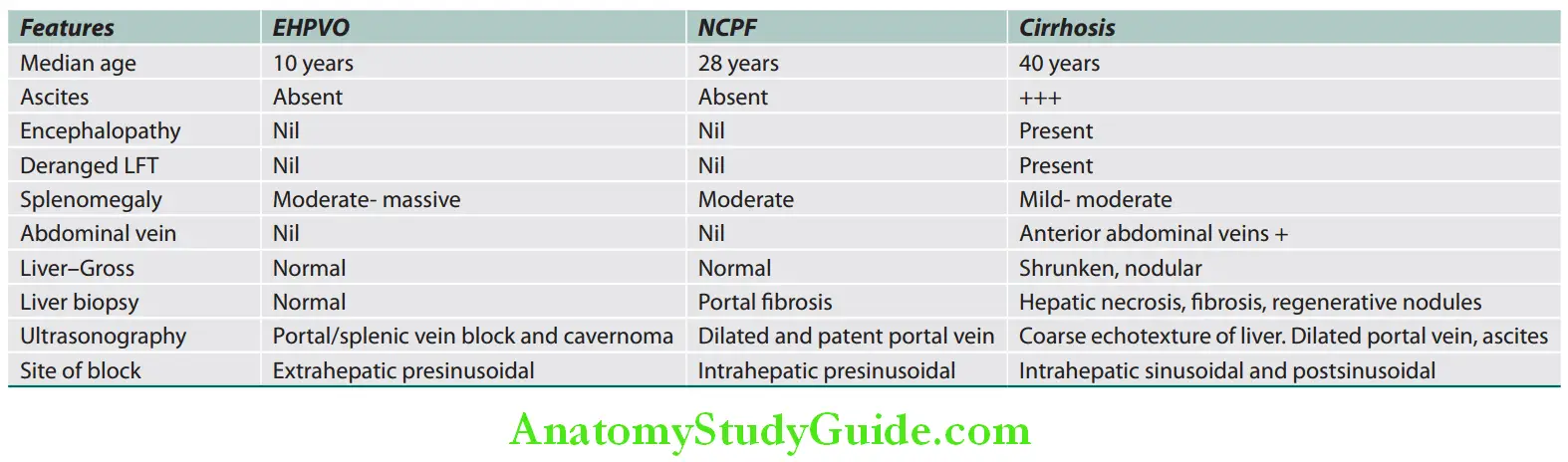
Common Causes of Painful Hepatomegaly:
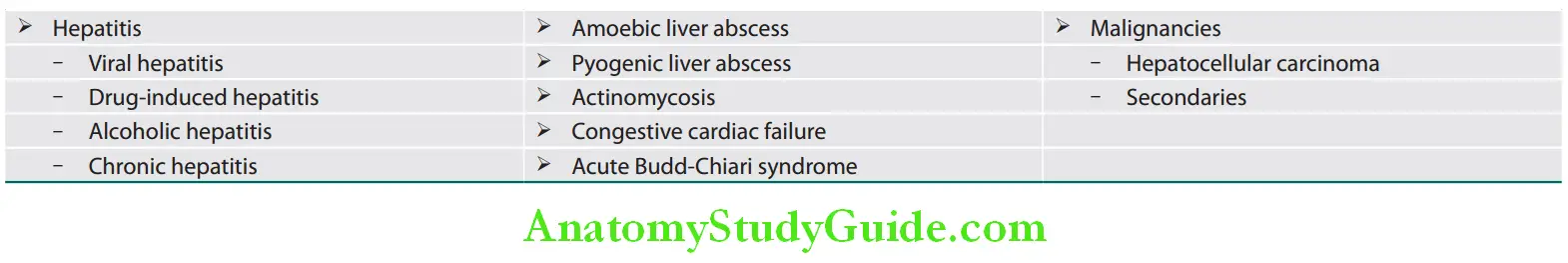
Question 72. What are the common causes of hepatomegaly with tenderness (tender hepatomegaly)?
(or)
Write short note on causes of pulsatile liver.
Answer:
Causes of Pulsatile Liver:
Causes of pulsatile liver:
- Tricuspid regurgitation (systolic)
- Tricuspid stenosis (diastolic)
- Aortic regurgitation
Causes of Splenomegaly:
Question 73. Enumerate the common causes of splenomegaly
Answer:
Classifiation of splenomegaly and their causes:
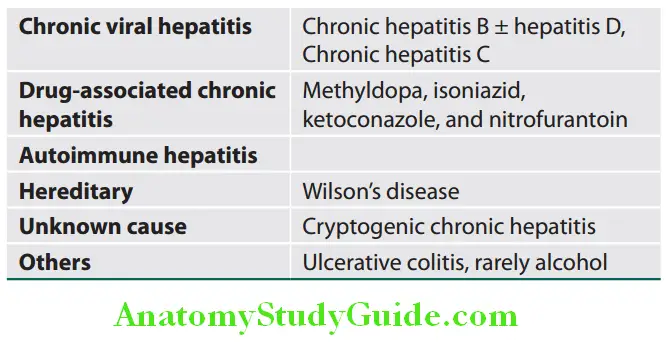
Causes of Hepatosplenomegaly:
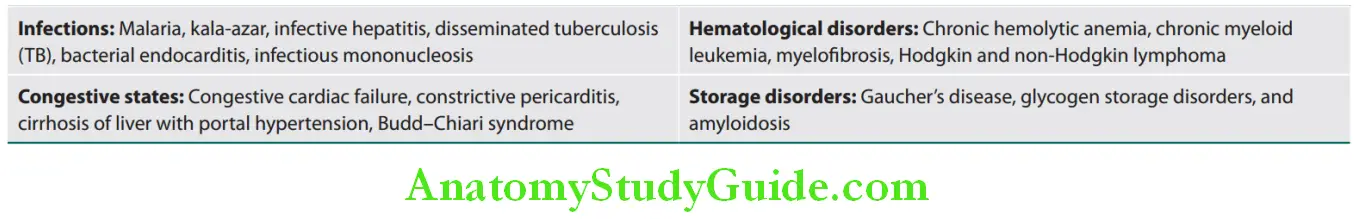
Causes of Hepatosplenomegaly with Lymphadenopathy:
- Lymphomas
- Infectious mononucleosis
- HIV
- Sarcoidosis
- Lymphocytic leukemia
- Disseminated tuberculosis
- Disseminated histoplasmosis
Hepatic Disorders Of Pregnancy:
Question 74. List the differential diagnosis for liver diseases during pregnancy
Answer:
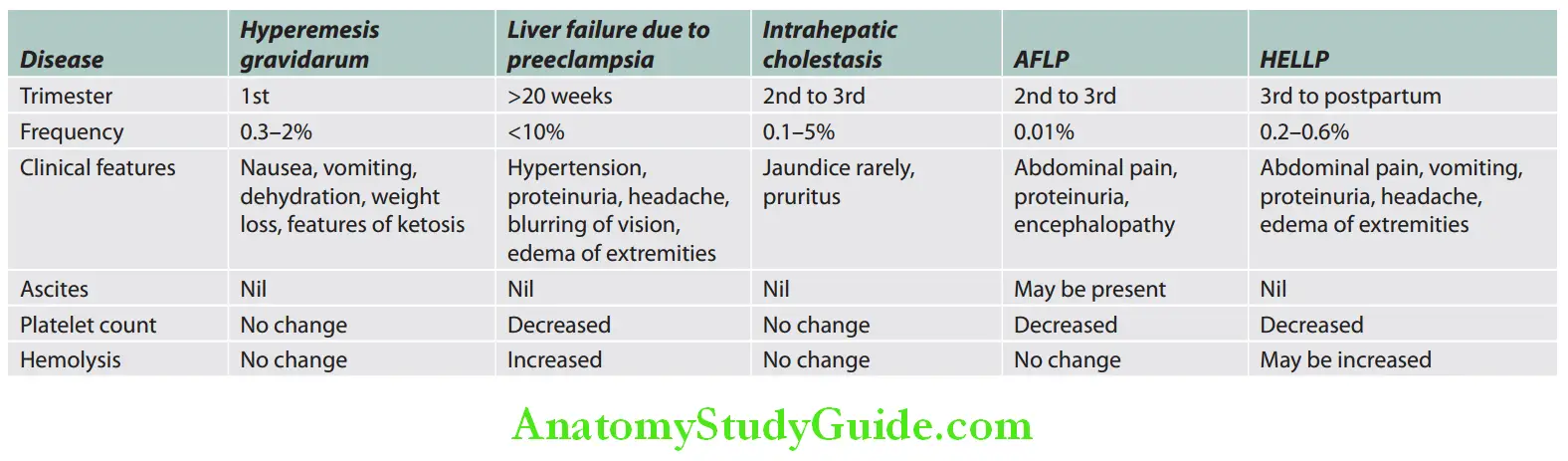
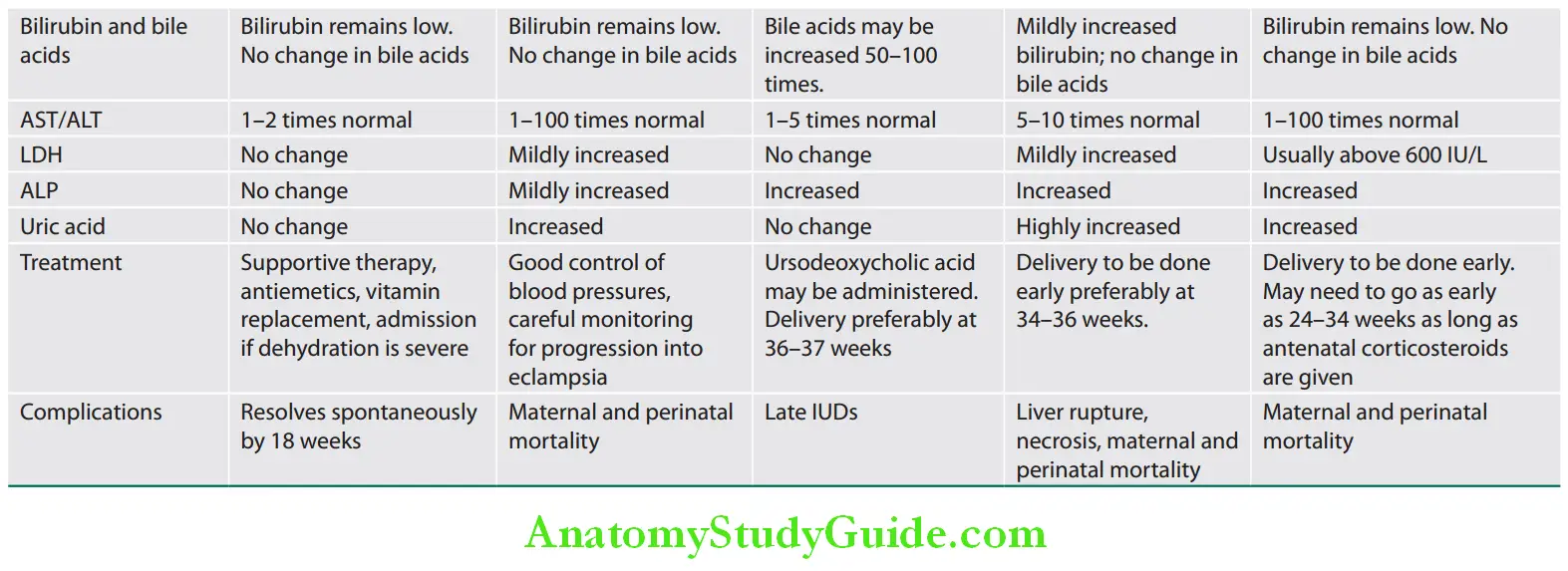
Leave a Reply