Hiv And Other Retroviruses
Retroviruses possess a unique enzyme ‘reverse transcriptase’ that directs the synthesis of DNA from the viral RNA after infection into a host cell. Only two genera are pathogenic to humans;HIV and HTLV.
Table of Contents
Human Immunodeficiency Virus (Hiv)
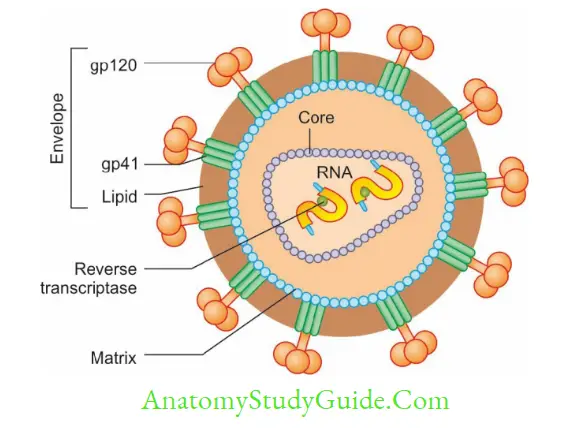
Morphology
Envelope: HIV and other lentiviruses are spherical and 80–110 nm in size, and possess an envelope; made up of a lipid layer in which two proteins are embedded:
- Glycoprotein 120 (gp 120) are projected as knob-like spikes on the surface
- Glycoprotein 41 (gp 41): They form anchoring transmembrane pedicles.
Read And Learn More: Micro Biology And Immunology Notes
Nucleocapsid: Capsid is icosahedral in symmetry, made up of core protein. Inside, there is an inner core which encloses:
- RNA: Two identical copies of single-stranded positive sense linear RNA
- Viral enzymes, such as reverse transcriptase, integrase and proteases are closely associated with HIV RNA.
HIV Genes and Antigens
- HIV contains 3 structural genes: gag, pol, and env, and 6 nonstructural or regulatory genes.
Structural Genes
Structural genes code for various components of the virus. - Gag gene: It is expressed as a precursor protein, p55 which is cleaved into 3 proteins: p18 (constitutes the matrix or shell antigen); p24, and p15 both of constitute the core antigens.
- Pol gene codes for viral enzymes, such as reverse transcriptase, protease, and integrase. It is expressed as a precursor protein, which is cleaved into proteins p31, p51, and p66.
- Env gene codes for the envelope glycoprotein (gp160), which is cleaved into gp120 and gp41.
Non-structural Genes
Non-structural genes regulate viral replication and are important in disease pathogenesis in vivo.
- That is a transcriptional transactivator gene
- Nef (negative factor gene), Rev (regulator of virus gene), Vif (viral infectivity factor gene)
- Vpu gene, Vpr gene, Vpx gene, and LTR (long terminal repeat) sequences.
- Antigenic Variation and Diversity of HIV
HIV shows extensive antigenic diversity because of undergoing high rates of mutation, especially the env gene, and due to the error-prone nature of the reverse transcriptase enzyme.
HIV Serotyping
Based on sequence differences in the env gene, HIV comprises two serotypes HIV-1 and 2.
HIV–1 is divided into three distinct groups (M, N, and O). Recently, group P has been identified.
- ‘M’is the dominant group worldwide. It comprises of ten subtypes or ‘clades’ (A-J).
- There are also ‘circulating recombinant forms’ or CRFs derived from recombination between different subtypes. For example, CRF01_AE is a recombination between subtypes A and E.
- The same infected host may have a group of closely related viral subtypes and/or CRF at a given time which are collectively called quasispecies.
- HIV-1 subtypes or clades do not vary in pathogenesis or biology, but they differ in geographical distribution and transmission.
- Geographical distribution:
- Subtype A is common in West Africa
- Subtype B is predominant in Europe, America, Japan, and Australia
- Subtype C is the MC form worldwide (47%). It is also the dominant form in South East Africa, India, and China
- In Cameroon (West Africa), all known HIV groups and subtypes are found. It is the place of origin of the virus.
- Transmission: Asian and African subtypes (C and E) are more readily transmitted heterosexually; whereas American strains (subtype B) preferentially spread through blood and homosexual contact.
HIV-2 comprises eight groups (A–H); they are confined to Africa and sometimes in other places including India. Group A is the MC form.
Pathogenesis
Mode of Transmission
Points to be noted:
- Transmission may occur at any time during pregnancy and breastfeeding but the risk is maximum during delivery.
- Risk is maximum if a mother is recently infected or has already developed AIDS.
- There is no evidence of HIV transmission by casual contact kissing or insect bite.
- Viral load is maximum in blood, genital secretions, and CSF; variable in breast milk and saliva; zero to minimal in other body fluids or urine.
- Saliva may contain inhibitory substances like fibronectin and glycoproteins, which prevent transmission of the virus.
Receptor Attachment and Fusion
- Main receptor: gp120 of HIV binds to the CD4 receptor on the host cell surface. CD4 molecules are mainly expressed on helper T-cells; but also on the surface of various other cells like monocytes, macrophages, Langerhans cells, astrocytes, keratinocytes, and glial cells.
- A second coreceptor is necessary for fusion of HIV by binding to gp120 and to gain entry into the host cell, for example.,
- CXCR4 molecules present on T-lymphocytes
- CCR5 molecules are present in cells of the macrophage lineage.
- DC-SIGN, a dendritic cell-specific lectin receptor can also bind to HIV-1 but does not mediate cell entry. Rather, it may facilitate the transport of HIV by dendritic cells to lymphoid organs where HIV replicates further in T-cells.
Replication
- After fusion, HIV undergoes penetration and uncoating → HIV RNA is converted to HIV
DNA by RT enzyme→ Preintegration complex is formed, comprised of linear dsDNA, gag matrix protein, accessory Vpr protein, and viral integrase which is transported into the host cell nucleus. - Integration: The viral dsDNA gets integrated into the host cell chromosome; mediated by viral integrase. The integrated virus is called provirus.
- Latency: In the integrated state, HIV establishes a latent infection for a variable period.
However, HIV is different from other latent viruses as it can replicate even in a latent state and is infectious to the neighboring cells.
Immunopathogenesis
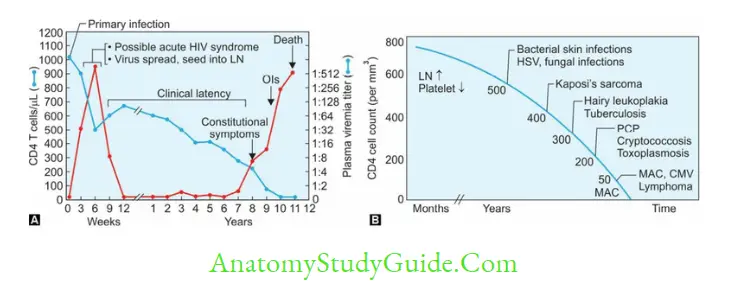
The natural course of the disease passes through the following stages:
- Acute HIV Disease or Acute Retroviral Syndrome
- Asymptomatic Stage
- Persistent Generalized Lymphadenopathy (PGL)
- Symptomatic HIV Infection (or AIDS-related complex, ARC)
- AIDS.
Clinical Diagnosis of HIV/AIDS
Classification systems are useful for tracking and monitoring the HIV epidemic and for providing clinicians and patients with important information about HIV disease stage and clinical management.
Two such systems are currently in use:
- CDC classification system (revised 1993): This system classifies HIV infection into nine stages based on associated clinical conditions and the CD4 T-cell count of the patient.
- WHO clinical staging of HIV/AIDS for adults (revised 2007) is based only on the clinical conditions. It is useful for resource-poor countries like India, where facilities for the CD4 Tcell count are not available widely.


Epidemiology of HIV/AIDS
Global Situation of HIV/AIDS
- Prevalence (number of cases per 100 population): At the end of 2016, about 36.7 million people were living with HIV (including 1.8 million children) with a global prevalence of 0.8% [0.7–0.9%] in adults.
- Sub-Saharan Africa remains most severely affected, with nearly one in every 25 adults living with HIV and accounting for nearly two-thirds of the people living with HIV worldwide.
HIV/AIDS Situation in India
- By the end of 2015, the adult HIV prevalence in India was reported as 0.26%.
- The number of PLHA (people living with HIV/AIDS) was over 2.1 million adults.
- Andhra Pradesh (undivided) was the worst affected state followed by Maharashtra and Karnataka in terms of PLHA.
- However, as far as prevalence (number of cases per 100 population) is concerned, Northeast states, such as Nagaland, Mizoram, and Manipur are worst affected.
- Highest prevalence: Manipur has the highest HIV prevalence of 1.15% (the only state to have an epidemic of HIV), followed by Mizoram (0.80%), Nagaland (0.78%), Andhra Pradesh and Telangana (0.66%), Karnataka (0.45%), Gujarat (0.42%) and Goa (0.40%).
- Other states to have HIV prevalence greater than the national prevalence (0.26%) are Maharashtra, Chandigarh, Tripura, and Tamil Nadu.
- High-risk group: HIV prevalence among female sex workers is 2.2%, men who have sex with men is 4.2%, Hijra/transgender people is 7.5% and IV drug abuser group is 9.9%.AIDS Control Organization
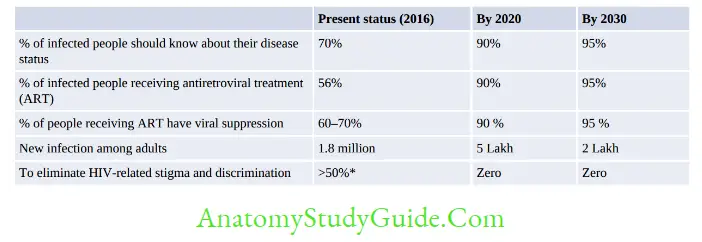
- UNAIDS: The Joint United Nations Program on HIV and AIDS (UNAIDS) is the main advocate for global action on HIV/AIDS. It has initiated the ‘Fast-Track strategy to end the AIDS epidemic by 2030’ (Table 4.6.2)
- NACO: National AIDS Control Organization (NACO) has been constituted to implement the
HIV/AIDS control program in India. It provides single national plan within one monitoring system - National Strategic Plan for HIV/AIDS and STI (2017–2024): NACO has launched the national strategic plan ‘Paving Way for an AIDS-Free India’; going in line with UNAIDS for ending the AIDS epidemic by 2030.
Laboratory Diagnosis

NACO Strategy for HIV Diagnosis
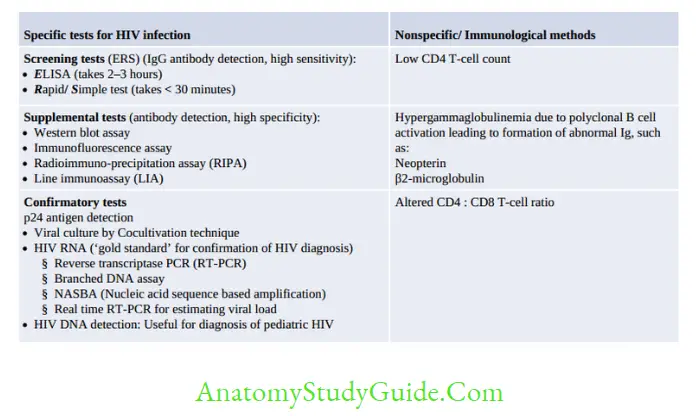
For resource-poor countries, it is impracticable to confirm the result of HIV screening tests by PCR or western blot as these assays are expensive and available only at limited centers.
NACO (National AIDS Control Organization, India) has formulated a strategic plan for HIV diagnosis.
Depending on the situation/condition, for which the test is done, the positive result of the first screening test should be either considered as such or confirmed by another one or two screening tests.
- The first screening test should be highly sensitive, whereas the second and third screening tests should have high specificity.
- The three screening tests should use different principles or different antigens. The same kit should not be used again.
- Supplemental or confirmatory tests should be used only when the screening test(s) results equivocal/intermediate.
- There are four NACO strategic plans/algorithms
- Strategy I: t is done for blood donors in blood banks. Only one test is done.
- Strategy IIa: It is done for seroprevalence or epidemiological purposes. Two tests are done.
- Strategy IIb: Purpose: It is followed for the diagnosis of HIV/AIDS in symptomatic patients. Two tests are done. If the second test is negative, then a third test is done for confirmation.
- Strategy III: It is done for the diagnosis of asymptomatic HIV patients, antenatal screening, and screening of patients awaiting surgeries. Three tests are done.
For indeterminate results of strategy IIB and III (i.e. first test positive but second or third test negative), the repeat test is done after 2–4 weeks and the sample should be sent to the reference center for confirmation by western blot or RT-PCR

Prognosis/Monitoring of HIV
Various tools available for monitoring the response to antiretroviral therapy include:
- CD4 T-cell count: Most commonly used
- HIV RNA load: Most consistent and best tool at present
- p24 antigen detection
- Neopterin and β2 microglobulin level
Note: Antibody levels are inconsistent during late stage due to immune collapse; hence not reliable for prognosis.
Diagnosis of Pediatric HIV
The routine screening methods (ELISA or rapid/simple tests) detect IgG antibodies.
They cannot differentiate between baby’s IgG or maternally transferred IgG, hence cannot be used for the diagnosis of pediatric HIV.
As all maternal antibodies would disappear by 18 months; IgG assays can only be performed after 18 months of birth.
Diagnosis of HIV in Window Period
Definition: Window period refers to the initial time interval between the exposure and appearance of detectable levels of antibodies in the serum.
- The antibodies appear in blood within 2–8 weeks after infection but usually become detectable after 3 to 12 weeks with the assays available presently. It can be as low as 22 days; when third-generation antibody detection kits with high sensitivity are used.
- p24 antigen detection (30% sensitive; by 4th generation ELISA): It can be detected by 12–26 days after infection.
- HIV RNA detection (by RT-PCR) is the best method—it detects HIV RNA around 10–14 days after infection.
Treatment
Antiretroviral Therapy (ART)

Antiretroviral therapy (ART) denotes the approved drugs used for the treatment of HIV/AIDS.
The drugs do not kill all the viruses or cure the disease. However, they have the following goals:
- Clinical goals: Prolongation of life, improvement in quality of life, and stoppage of the progression of HIV infection to AIDS
- Virological goals: Greatest possible reduction in the viral load as long as possible
- Immunological goals: Immune reconstitution; both quantitative and qualitative improvement
- Transmission goals: Reduction of HIV transmission in individuals.
Initiation of ART: NACO guidelines recommended the initiation of ART based on
(1) CD4 T cell count,
(2) the WHO stage of the disease and
(3) associated opportunistic infections (OIs) such as TB, HBV, HCV, or
(4) pregnancy/breastfeeding women.
However, according to the revised NACO guideline 2017, ART has to be started in all patients irrespective of CD4 count, clinical stage, age, population or associated OIs.
Highly Active Antiretroviral Therapy (HAART)
HAART refers to the use of a combination of at least three antiretroviral drugs to maximally suppress HIV and stop the progression of the disease. Monotherapy with a single drug is contraindicated due to inefficacy and the chance of the development of resistance.
NACO Recommended HAART Regimen
First-line regimen: NACO recommends including three drugs (2NRTIs/NtRTI + 1NNRTI) as the first-line regimen.
- TLE (tenofovir 300 mg + lamivudine 300 mg + efavirenz 600 mg) is the NACO recommended regimen. It should be started for all new patients as single dose daily regimen
- Older regimens: If the patients are already on a different ART regimen and responding well —then the same regimen can be continued.
-
- ZLN (Zidovudine + Lamivudine + Nevirapine)
- ZLE (Zidovudine + Lamivudine + Efavirenz)
- TLN (Tenofovir + Lamivudine + Nevirapine).
- Monitoring: CD4 count and viral load have to be monitored every 3–6 months to monitor the response to treatment.
Second-line regimen: If the patient is not responding to the primary regimen or develops toxicity; then switch over to a protease inhibitor-based regimen (1 PI + 2NRTIs/NtRTIs). Examples include tenofovir + lamivudine + lopinavir or ritonavir.
Opportunistic Infections (OIs)
OIs should be adequately treated with appropriate antimicrobial agents before starting ART.
Cotrimoxazole prophylaxis (for prevention of Pneumocystis pneumonia).
Cotrimoxazole may be initiated in the following scenarios:
- HIV-infected adults with CD4 count <200/cm
- WHO clinical stage 3 or 4 irrespective of CD4 count.
Problems About the Use of ART
- Adverse side effects, High cost of ARTs, Limited therapeutic options
- Risk of development of drug resistance and dissemination of resistant virus
- IRIS: Immune reconstitution inflammatory syndrome (IRIS) occurs in some cases of AIDS during the recovery phase following the start of ART.
As the viral load decreases, the immune system begins to recover but then responds to a previously acquired opportunistic infection with an overwhelming inflammatory response that paradoxically makes the symptoms of infection worse. - Note: Post-exposure prophylaxis (PEP) for HIV has been described as a part of Needle stick injury in Chapter 7.2.
Human T-Cell Lymphotropic Virus (Htlv)
Two important members are HTLV-I and II. HTLV-I is only pathogenic, as described below.
- Transmission by: (1) from mother to child via breast milk (most common); (2) homosexual;(3) infected blood
- TargeT-cells: It infects CD4 T-cells; but occasionally also infects CD8 T-cells, dendritic cells, and B-cells.
- Target receptor: Viral gp binds to hosT-cell receptor GLUT1(Human glucose transporter protein-1)
- The tax gene of HTLV-I acts as a transactivator and is responsible for its oncogenicity
- Distribution-HTLV-I is endemic in certain parts of Japan (10% prevalence) and the Caribbean basin of Africa
- Genotypes: It has 7 genotypes; type-A is the most common, while others are found only in central Africa.
- Clinical manifestations: HTLV-I is a potential human oncogenic virus:
- Adult T-cell leukemia/lymphoma
- Cutaneous T-cell lymphoma
- Tropical spastic paraparesis
- Autoimmune features, such as inflammatory disease, uveitis, and arthropathies.
Leave a Reply