Immunity
Question 1. Define innate immunity. What are the components of innate immunity?
Answer:
- Definition: Innate immunity refers to the mechanisms that are ready to react to infections even before they occur
Components of innate immunity include:
- Epithelial barriers: Epithelia of the skin, gastrointestinal and respiratory tracts, which prevent the entry of microbe
- Monocytes and neutrophils: These are phagocytes in the blood that can be recruited to the site of infection
- Dendritic cells: These are antigen-presenting cells and display the microbial peptides to T-lymphocytes
- Natural killer cells: Protects against viruses
- Mast cells and proteins of the complement system
Read and Learn More Preparatory Manual of Pathology Question and Answers
Question 2. Write a short note on toll-like receptors.
Answer:
Toll-like receptors (TLRs)
- Act as a cellular receptor for microbes in innate immunity
- Are present in the plasma membrane and endosomal vesicles of the cell
- Microbe when comes in contact with a cell, is recognized by these toll-like receptors
- Following the microbe recognition, toll-like receptors send signals that result in the activation of NF-κB and interferon regulatory factors
- NF-κB stimulates the synthesis and secretion of cytokines, which recruits neutrophils
- Interferon regulatory factors (IRFs) produce antiviral cytokines, like type I interferon
- Both of these result in the control of microbial infection
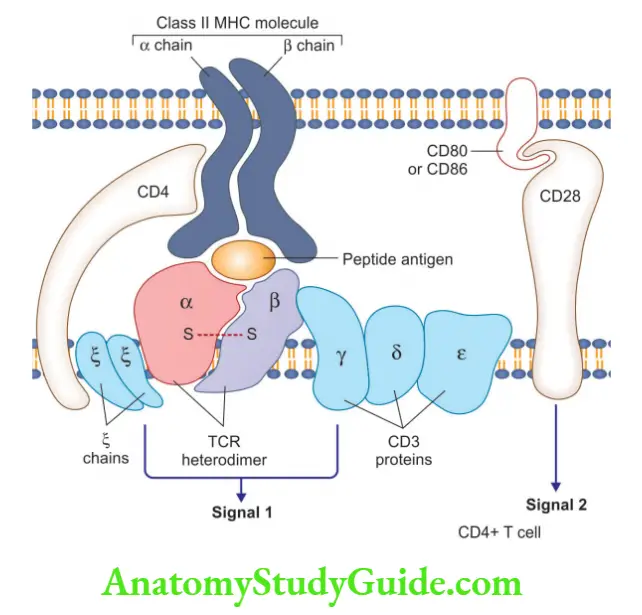
Question 3. Write a short note on the T cell receptor complex.
Answer:
- T-lymphocytes are classified into helper T lymphocytes, cytotoxic T lymphocytes (CTLs), and regulatory T lymphocytes
T cell receptor complex comprises:
- TCR heterodimer consists of α and β chains that recognize antigens, expressed by MHC molecules on antigen-presenting cells (APCs)
- CD3 complex and ζ chains of the T cell receptor complex, initiate the activating signals
- Other than CD4, T cells also express CD8 molecules
- CD4+ T cells: In response to the antigen, it stimulates cytokine production
- CD8+ T cells: It functions as cytotoxic (killer) T cells
- Function: T cell recognizes specific cell-bound antigens using an antigen-specific TCR
Question 4. Write briefly on natural killer cells.
Answer:
Natural killer cells
- Also called large granular lymphocytes, as they contain abundant azurophilic granules
- They can kill infected cells and tumor cells, without prior exposure to activation by these microbes/tumors
- They act as an early line of defense against viral infections and tumors
- CD16 and CD56 are used to identify NK cells
- Antibody-dependent cell-mediated cytotoxicity: CD16 on NK cells is an Fc receptor for IgG, and confers NK cells the ability to lyse IgG-coated target cells
- NK cells secrete interferon-γ (IFN-γ), which activates macrophages to destroy ingested microbes
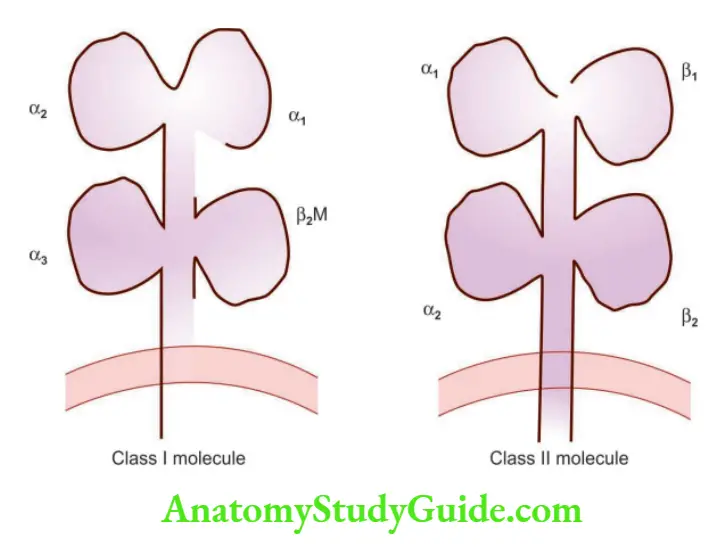
Question 5. Write a note on the major histocompatibility complex and its structure.
Answer:
Major histocompatibility complex (MHC)
- Display peptide fragments of proteins for recognition by T cells (antigen-specific)
- Also called human leukocyte antigens (HLA)
- Genes encoding HLA are located on chromosome 6
Class I MHC molecules
- Are expressed on all nucleated cells and platelets
- They are heterodimers consisting of “α or heavy chain” linked to a smaller peptide chain called “β2-microglobulin”
- α chains are encoded by three genes: HLA-A, HLA-B, and HLA-C
- α chain is divided into three domains: α1, α2, and α3
- α1 and α 2 domains form a cleft, or groove, where peptides bind
- α3 domain has a binding site for CD8+ T cells (cytotoxic T-lymphocytes)
- As CD8+ T cells recognize the peptides presented by class I MHC molecules, CD8+ T cells are class I MHC-restricted
Class II MHC molecules
- Encoded in a region called HLA-D, which has three sub-regions: HLA-DP, HLA-DQ, and HLA-DR
- They are heterodimers composed of α chain and β chain, and both have two domains designated α1 and α2, and β1 and β2
- Peptide-binding cleft formed by an interaction of the α1 and β1 domains
- β2 domain has a binding site for CD4
- As CD4+ T cells recognize peptides/antigens presented by class II MHC molecules, CD4+ T cells are class II MHC restricted
Class III MHC molecules
- Encodes complement components and the cytokines (TNF and lymphotoxin)
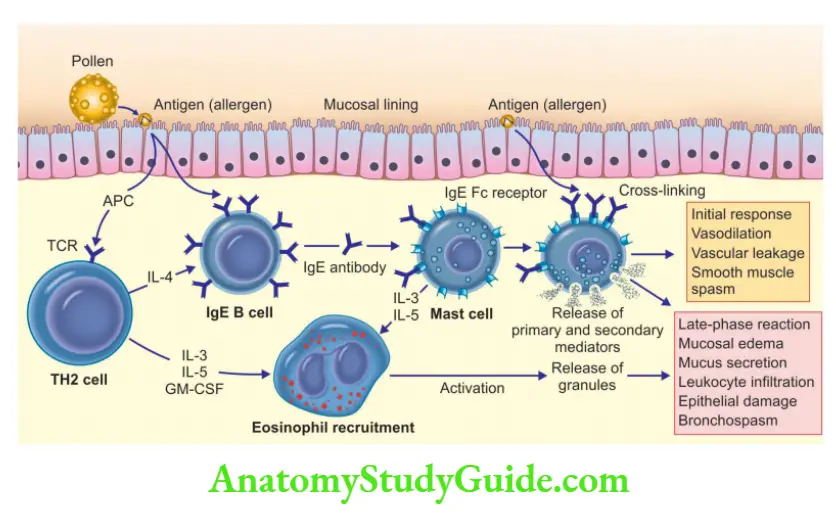
Question 6. Define hypersensitivity. Discuss in detail type I hypersensitivity reactions.
Answer:
- Hypersensitivity: Implies an excessive or harmful reaction to an antigen
Immediate (type I) hypersensitivity
- Definition: Rapid immunologic reaction, which occurs within minutes, after the antigen combines with the antibody bound to the mast cells, seen in individuals, who are previously sensitized to the antigen
Two well-defined phases
1. Immediate reaction
- Occurs within minutes after exposure to an allergen and subsides within a few hours
- Characterized by vasodilatation, vascular leakage, and smooth muscle spasm
2. Late-phase reaction
- Occurs 2–24 hours later
- It is characterized by the infiltration of tissues with eosinophils, neutrophils, basophils, monocytes, and CD4+ T cells with resultant mucosal epithelial cell damage
- Helper T cells are classified into TH1 cells, TH2 cells, and TH17 cells
- TH2 cells play a role in type I hypersensitivity reactions
Role of TH2 cells
- Antigen when enters the body is captured by dendritic cells, which present it to naïve CD4 + T cells
- Naïve CD4+ T cells, in response to the antigen, release IL-4 and differentiate into TH2 cells
- TH2 cells produce cytokines IL-4, IL-5, and IL-13
- IL-4 acts on B cells to stimulate IgE production, which binds to the mast cells (mast cells express FcRI, specific for the Fc portion of IgE)
- IgE-coated mast cells, if exposed to similar antigens, result in mast cell activation and there occurs release of mediators, which bring about the clinical expression of immediate hypersensitivity reactions
Mediators of type I hypersensitivity reactions:
1. Preformed mediators: Present within mast cell granules, and are divided into 3 categories:
1. Vasoactive amines: Like histamine which brings about smooth muscle contraction, increased vascular permeability, and increased mucus secretion
2. Enzymes: Proteases
3. Proteoglycans: Heparin, chondroitin sulfate
2. Lipid Mediators:
- Phospholipase A2 in mast cells converts membrane phospholipids to arachidonic acid
1. Arachidonic acid metabolites
- Leukotrienes B4, C4, D4:
- LTC4 and D4 are the most potent vasoactive agents, increases vascular permeability, bronchial smooth muscle contraction
- LTB4 is chemotactic for neutrophils, eosinophils, and monocytes
- Prostaglandin D2: Results in bronchospasm and increased mucus secretion
2. Platelet-activating factor (PAF):
- Platelet aggregation, release of histamine, bronchospasm, increased vascular permeability, and vasodilation
3. Cytokines:
- TNF, IL-1, and chemokines: Leukocyte recruitment (in late phase reaction)
- IL-4: Amplifies TH2 response
Examples of type I hypersensitivity reactions: Anaphylaxis, bronchial asthma, allergic rhinitis, hay fever, food allergies
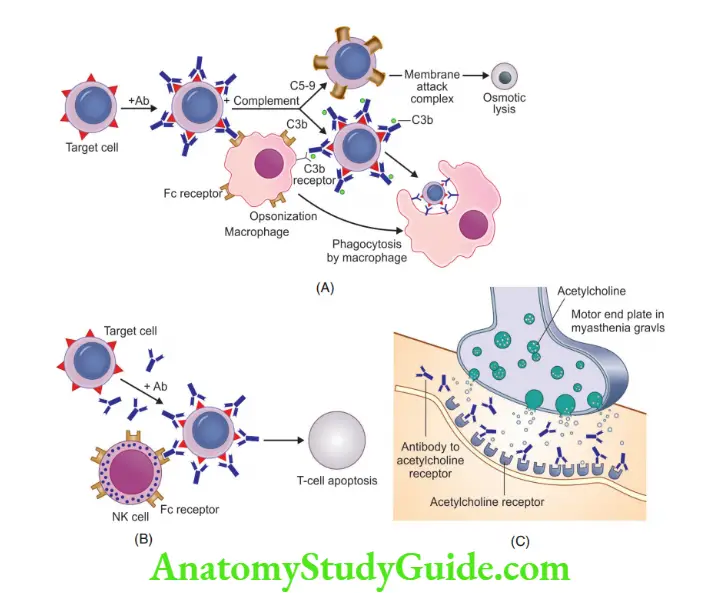
Question 7. Discuss in detail Type II Hypersensitivity reactions with examples.
Answer:
Antibody-mediated (type II) hypersensitivity
- Caused by antibodies that react with antigens present on the cell surface or in the extracellular matrix
Antibody-mediated mechanisms
1. Opsonization and phagocytosis:
- IgM or IgG antibodies on the cell surface, activate the complement system by classical pathway, resulting in deposition of C3b or C4b on the cell surface, which is recognized by phagocytes, resulting in phagocytosis of the opsonized cells
- Cells opsonized by IgG antibodies are recognized by phagocyte Fc receptors, which are specific for the Fc portions of IgG subclasses
- For example Transfusion reactions, erythroblastosis fetalis, autoimmune hemolytic anemia, agranulocytosis, thrombocytopenia
2. Inflammation
- Antibodies deposited on the basement membrane stimulate the complement system and result in the activation of complement by-products like C5a and C3a
- C5a and C3a result in the activation of neutrophils, monocytes, and increased vascular permeability
- Activated leucocytes release mediators which damage the basement membrane, collagen, elastin, and cartilage
3. Cellular dysfunction
- Antibodies are formed against cell surface receptors, which impair or dysregulate its function, for example
- In Myasthenia gravis, antibodies are formed against the ACh receptor in the motor end plate of skeletal muscles, blocking neuromuscular transmission and muscle weakness
- Graves’ disease, antibodies against the thyroid-stimulating hormone receptor on thyroid epithelial cells stimulate the cells, resulting in hyperthyroidism
Examples of type II hypersensitivity reactions
- Autoimmune hemolytic anemia, autoimmune idiopathic thrombocytopenic purpura, pemphigus vulgaris, vasculitis caused by ANCA, Goodpasture syndrome, acute rheumatic fever, myasthenia gravis, Graves’ disease, insulin-resistant diabetes
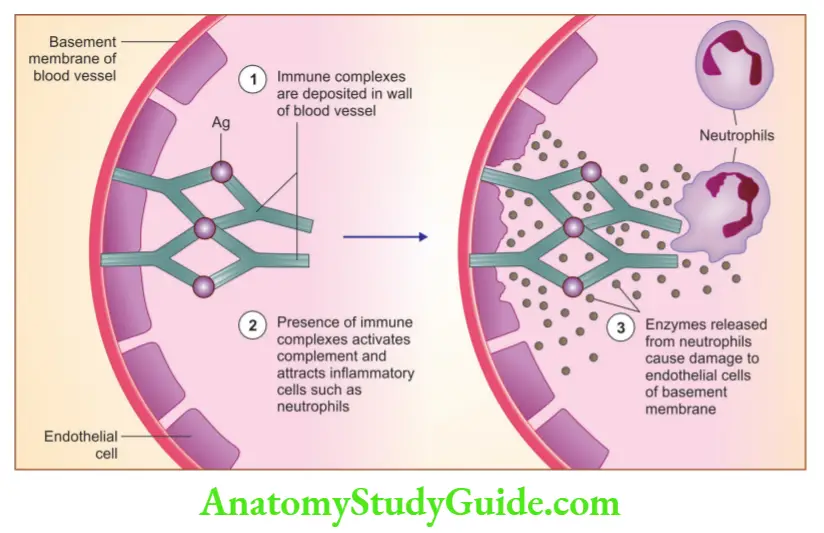
Question 8. Write a short note on type III hypersensitivity reactions.
Answer:
Immune complex-mediated (type III) hypersensitivity
- Antigen-antibody complex gets deposited in the vessel wall and produces tissue damage by eliciting inflammation
- The antigen can be exogenous or endogenous (autoimmunity)
- Can be systemic (immune complexes in circulation) or can involve kidneys, joints, and small blood vessels
Two types
1. Systemic immune complex disease
Acute serum sickness is the prototype
Pathogenesis is divided into three phases:
- Formation of antigen-antibody complexes in the circulation
- Deposition of immune complexes: Most commonly affects glomeruli and joints
- Inflammation and tissue injury by immune complexes
2. Local immune complex disease (Arthus reaction)
- Results from acute immune complex vasculitis, elicited in the skin
- Can be produced experimentally by intra-cutaneous injection of an antigen in a previously immunized animal
Examples of type III hypersensitivity reaction
- SLE, post-streptococcal glomerulonephritis, polyarteritis nodosa, reactive arthritis, serum sickness, and Arthus reaction
Question 9. Discuss type IV hypersensitivity reaction with examples.
Answer:
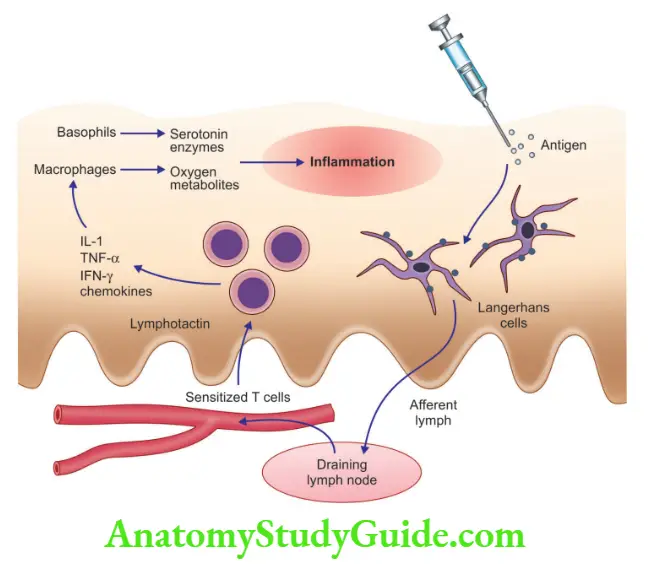
T cell-mediated (type IV) hypersensitivity: Can be induced by CD4+ T cells and CD8+ T cells
1. CD4+ T cell-mediated inflammation
Cytokines produced by T cells induce inflammation
Presents as delayed-type hypersensitivity (DTH)
Example of DTH
1. Tuberculin reaction:
- Produced by intra-cutaneous injection of purified protein derivative (PPD) tuberculin, in previously sensitized individuals, reddening and the duration of the site appears in 8–12 hours, reach a peak in 24–72 hours, and slowly subside
2. Contact dermatitis
3. Rheumatoid arthritis, multiple sclerosis, inflammatory bowel disease
2. CD8+ T cell-mediated cytotoxicity
- CD8+ cytotoxic T-lymphocytes (CTLs) kill antigen-expressing target cells
- Examples: Seen in type I DM, graft rejection, and reactions against viruses
Question 10. Write a note on the mechanism of autoimmune diseases.
Answer:
First, we need to understand the concept of tolerance!
- Tolerance means unresponsiveness to self-antigens, which is a fundamental property of the immune system
- In autoimmune diseases, there is a breakdown of tolerance Mechanisms of self-tolerance:
Two types
- Central tolerance: In the thymus and bone marrow, immature lymphocytes that recognize self-antigens are killed by apoptosis
- Peripheral tolerance: Cells that escape central organs, come into the peripheral blood, and are inactivated in the peripheral lymphoid system
1. Central tolerance
- Immature T and B lymphocytes, during their maturation in the thymus (T cells) and bone marrow (B cells), recognize self-antigens and kill them by
1. Negative selection/ deletion: In the thymus, immature lymphocytes encounter the antigen cells die of apoptosis
2. Receptor editing: B cells recognize self-antigens in the bone marrow, and begin to express new antigen receptors, not specific for self-antigens
2. Peripheral tolerance
- Mechanisms that silence potentially auto-reactive T and B cells in peripheral tissues
1. Energy
- Functional inactivation of lymphocytes, that recognize self-antigens
2. Suppression by regulatory T cells
- Regulatory T cells function to prevent immune reactions against self-antigens
3. Deletion by apoptosis
- T cells that recognize self-antigens may die by apoptosis, which can occur due to activation of pro-apoptotic molecule Bim or activation-induced death of CD4+ T cells and B cells by Fas-Fas ligand system
Mechanisms/pathogenesis of autoimmune diseases
1. Role of susceptibility genes
- Most autoimmune diseases are complex multigenic disorders
- Examples: HLA-B27 association with ankylosing spondylitis, non-MHC genes like polymorphisms in PTNP22 associated with type I DM, rheumatoid arthritis, polymorphisms in NOD2 associated with Crohn disease
2. Role of Infections
- Infection induces co-stimulators on antigen-presenting cells, which result in the activation of T cells, resulting in tissue destruction
- Molecular mimicry: Microbes express antigens that resemble self-antigen, which results in the activation of self-reactive lymphocytes, for example, rheumatic heart disease
Question 11. Enumerate revised criteria for the classification of systemic lupus erythematosus (SLE).
Answer:
Revised criteria for the classification of systemic lupus erythematosus
- Malar rash: Erythema over malar eminences
- Discoid rash: Erythematous raised patches with scaling and plugging
- Photosensitivity: Rash due to sunlight
- Oral ulcers: Oral or nasopharyngeal ulceration
- Arthritis: Non-erosive arthritis involving two or more peripheral joints
- Serositis: Pleuritis or pericarditis
- Renal disorder: Persistent proteinuria > 0.5 gm% or cellular casts
- Neurologic disorder: Seizures, psychosis
- Hematological disorder: Hemolytic anemia with reticulocytosis or leucopenia or lymphopenia or thrombocytopenia
- Immunologic disorder:
Anti-DNA antibody or anti-Sm antibody or positive finding of antiphospholipid antibody syndrome - Antinuclear antibody: Abnormal titer of ANA by immunofluorescence
Note: A person is said to have SLE if any four or more of the 11 criteria are present.
Question 12. Discuss antibodies specific to SLE and the pathogenesis of the disease.
Answer:
Antinuclear antibodies (ANAs) in SLE are directed against nuclear antigens and grouped into 4 categories:
- Antibodies to DNA
- Antibodies to histone
- Antibodies to non-histone proteins bound to RNA
- Antibodies to nucleolar antigens
Etiology and pathogenesis of SLE
1. Genetic factors
- HLA-DQ locus alleles are responsible for the production of anti-double-stranded DNA, anti-Sm, and antiphospholipid antibodies
- Lupus patients have inherited deficiencies of C2, C4, or C1q which impairs the removal of circulating immune complexes by the mononuclear phagocyte system and thus favoring tissue deposition
2. Immunologic factors
- Nuclear DNA and RNA in antigen-antibody complexes stimulate B-lymphocytes resulting in increased production of anti-nuclear antibodies
3. Environmental factors
- Exposure to ultraviolet (UV) light, induces apoptosis in cells, these apoptotic cell become immunogenic and exacerbate the disease
- UV light stimulates keratinocytes, which release IL-1 and promote inflammation
- Drugs: Hydralazine, procainamide, and D-penicillamine can induce an SLE-like response
Question 13. Discuss the renal morphology in SLE.
Answer:
Lupus nephritis
- Glomerular lesions occur due to immune complex deposition Six patterns of glomerular disease are seen in SLE
Six patterns of glomerular disease are seen in SLE
1. Minimal mesangial lupus nephritis (class I)
- Least common, immune complex deposition in the mesangium, confirmed by immunofluorescence microscopy (IF) and EM (electron microscopy)
2. Mesangial proliferative lupus nephritis (class II)
- Mesangial cell proliferation and accumulation of mesangial matrix
3. Focal lupus nephritis (class III):
- Involvement of fewer than 50% of all glomeruli
- Lesions may be segmental (affecting only a portion of the glomerulus) or global (involving the entire glomerulus)
- Glomeruli show swelling and proliferation of endothelial and mesangial cells with leukocyte accumulation, papillary necrosis, and hyaline thrombi
4. Diffuse lupus nephritis (class IV)
- The most common and severe form of lupus nephritis
- Half or more of the glomeruli are affected
- Involved glomeruli show the proliferation of endothelial, mesangial, and epithelial cells
- Wire loop structures: Sub-endothelial immune complex deposits leading to circumferential thickening of the capillary wall on light microscopy
5. Membranous lupus nephritis (class V)
- Diffuse thickening of the capillary walls due to deposition of sub-epithelial immune complexes
- Severe proteinuria or nephrotic syndrome
6. Advanced sclerosing lupus nephritis (class VI)
- Sclerosis of more than 90% of the glomeruli represents end-stage renal disease
Question 14. What is an LE cell?
Answer:
Antinuclear antibodies (ANAs) are seen in SLE patients
- ANAs cannot penetrate the intact cell, but in tissues, nuclei of damaged cells react with ANAs, which now lose their chromatin pattern and become homogenous to produce “LE bodies” or “hematoxylin bodies”
- LE cell is any phagocytic leukocyte (neutrophil or macrophage), that has engulfed the denatured nucleus of an injured cell
Question 15. Write a note on transplant rejection.
Answer:
- Rejection: Mechanism by which the recipient’s immune system recognizes the graft as foreign and attacks it
Mechanism of rejection
- T lymphocytes and antibodies produced against graft antigens react against and destroy tissue graft
a. Lymphocyte-mediated destruction
- T cell-mediated rejection is brought about by CD4 and CD8 T cells, which recognize the graft antigens, presented by donor antigen-presenting cell
b. Antibody-mediated destruction
- Brought about by preformed antibodies or antibodies produced due to donor antigens
Morphology
- Rejection of kidney grafts is classified into Hyperacute, acute, and chronic forms
Question 16. Write a short note on hyper-IgM syndrome.
Answer:
Hyper-IgM syndrome
- Patients can produce IgM antibodies, but not IgG, IgA, or IgE antibodies
- CD4+ helper T cells express CD40 molecule
- Because of the expression of the CD40 molecule, CD4+ T cell recognizes CD40L (ligand) on B cells, macrophages, and dendritic cells, resulting in the activation of B-cells
- In hyper IgM syndrome, CD40L is mutated
- The serum of these patients contains normal or elevated levels of IgM, but no IgA or IgE and extremely low levels of IgG, although the number of B and T cells is normal
- Presents as recurrent pyogenic infections, Pneumocystis Kirov pneumonia
- IgM antibodies react with blood cells, giving rise to autoimmune hemolytic anemia, thrombocytopenia, and neutropenia
Question 17. Write a note on opportunistic infections and neoplasms in AIDS.
Answer:
AIDS: Defining opportunistic infections and neoplasms in HIV-infected patients:
1. Infections
1. Protozoal and helminthic infections
- Cryptosporidiosis or isosporidiosis (enteritis)
- Pneumocystosis (pneumonia or disseminated infection)
- Toxoplasmosis (pneumonia or CNS infection)
2. Fungal infections
- Candidiasis (esophageal, tracheal, or pulmonary)
- Cryptococcosis (CNS infection)
- Coccidioidomycosis (disseminated)
- Histoplasmosis (disseminated)
3. Bacterial infections
- Mycobacteriosis (Mycobacterium avid-intracellular, Mycobacterium tuberculosis)
- Nocardiosis (pneumonia, meningitis, disseminated)
- Salmonella infections disseminated
4. Viral infections
- Cytomegalovirus (pulmonary, intestinal, retinitis, or CNS infections)
- Herpes simplex virus
- Varicella-zoster virus
- Progressive multifocal leukoencephalopathy
2. Neoplasms
- Kaposi sarcoma
- Primary lymphoma of the brain
- Invasive cancer of the uterine cervix
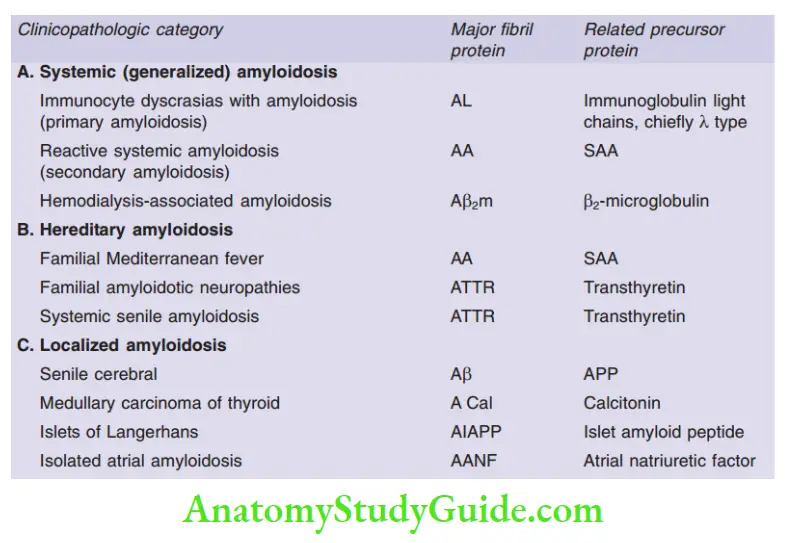
Question 18. Discuss in detail the pathogenesis and classification of amyloidosis and the staining characteristics of amyloid.
Answer:
- Amyloid: Pathologic proteinaceous substance, which on hematoxylin and eosin (H&E) stain, appears as an amorphous, eosinophilic, hyaline, extracellular substance
- Pathogenesis of amyloidosis:
- Results from abnormal folding of proteins, which are deposited as fibrils in extracellular tissues and disrupt normal function
- Normally, misfolded proteins are degraded intracellularly in proteasomes or extracellularly by macrophages
- In amyloidosis, these mechanisms fail and misfolded protein accumulates outside cells
Classification of Amyloidosis
The staining pattern of amyloid
- The most common stain used is Congo red
- Congo red, under ordinary light, imparts pink or red color to amyloid
- Under polarized light, Congo red-stained amyloid shows apple-green birefringence
Question 19. Write a note on the pathology of the spleen in amyloidosis and mention the physical properties of amyloid.
Answer:
Morphology of spleen in amyloidosis:
- Results in splenomegaly
- Sago spleen: Amyloid deposits are limited to the splenic follicles, producing tapioca-like granules on gross inspection
- Lardaceous spleen: Amyloid deposits in the walls of the splenic sinuses and in the red pulp, producing large map-like areas
The physical nature of amyloid
- Electron microscopy: Amyloid appears as continuous, non-branching fibrils with a diameter of approximately 7.5 to 10 nm
- X-ray crystallography: Amyloid shows a characteristic cross-β-pleated sheet conformation
Leave a Reply