Inflammation
Definition Inflammation: Inflammation is a complex local response of the living vascularized tissues to injury (infections and damaged tissues) and mainly consists of responses of blood vessels and leukocytes.
Table of Contents
It brings cells and molecules of host defense from the circulation to the sites of injury, in order to eliminate the offending agents/cause.
Acute Inflammation
Define inflammation.
Mention the types of inflammation. List the differences between acute and chronic inflammation.
Definition of Acute Inflammation: Inflammation is a complex local response of the living vascularized tissues to injury (infections and damaged tissues) and mainly consists of responses of blood vessels and leukocytes.
It brings cells and molecules of host defense from the circulation to the sites of injury, in order to eliminate the offending agents/cause.
Read and Learn More Preparatory Manual of Pathology Question and Answers
Protective vs harmful:
- Protective response: Inflammation is fundamentally a protective/defensive response. It helps in the removal/destruction of the initial cause of cell injury (for example, Microbes, toxins) and the consequences of such injury (for example, Necrotic cells).
- Harmful consequences of inflammation: Inflammatory reactions to infections often
cause local tissue damage and its associated signs and symptoms (for example, Pain and functional disturbances).- Usually, these harmful consequences are self-limited and resolved as the inflammation subsides. However, this normally protective inflammatory reaction may be the cause of the disease and may bring about damage to the tissues.
- In many diseases, the inflammatory reaction may be misdirected (for example, Against own/self-tissues in autoimmune diseases), occurs against normally harmless environmental substances (for example,In allergies), or is inadequately controlled (for example, Rheumatoid arthritis, atherosclerosis, hypersensitivity reactions).
Read And Learn More: Pathology for Dental Students Notes
Type of inflammation:
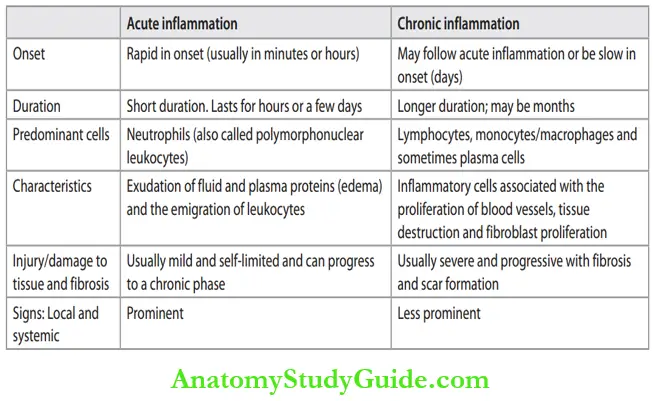
Inflammation may be divided into acute or chronic. Differences between acute and chronic inflammation are listed in Table.
Differences between acute and chronic inflammation:
Cardinal Signs of Inflammation:
Mention the cardinal signs of inflammation and its mechanism
- The four cardinal signs of inflammation as mentioned by Celsus are listed in Table 3.2.
- A fifth clinical sign, loss of function (function least), was later added by Rudolf Virchow.
Cardinal signs of inflammation:

Causes Of (Stimuli For) Acute Inflammation
Mention the various causes of acute inflammation.
- Infections (bacterial, viral, fungal, and parasitic) and microbial toxins
- Tissue necrosis:
- Ischemia, for example, Myocardial infarction
- Physical Agents
- Mechanical trauma, for example, Blunt/penetrating/crush injuries
- Thermal injury, for example, Burns or frostbite
- Radiation
- Electric shock
- Sudden changes in atmospheric pressure
- Chemical injury, for example, Strong acids and alkalies, insecticides, and herbicides
- Foreign bodies, for example, Sutures, talc
- Immune reactions:
- Hypersensitivity reactions
- Autoimmune diseases.
Sequence Of Events In Acute Inflammation
- Explain the sequential vascular changes/reactions of blood vessels/hemodynamic changes in acute inflammation. Acute inflammation has two major components, namely Reactions of blood vessels (vascular changes), and
- Reactions of leukocytes (cellular events) are characterized by leukocyte recruitment to sites of inflammation and their activation to eliminate the causative agent.
Reactions Of Blood Vessels (Vascular Changes)
- Purpose: To deliver the circulating cells (leukocytes), flids, and plasma proteins from the circulation to sites of infection or tissue injury.
- The reactions of blood vessels in acute inflammation consist of changes in the vascular flw and caliber and increased vascular permeability.
Changes in Vascular Flow and Caliber:
- Vasodilatation: It is the earliest feature of acute inflammation; sometimes it follows a transient constriction of arterioles. Vasodilation first affects the arterioles followed by
the opening of new capillary beds in the area.- Effect: Result is increased blood flow → Local heat and redness.
- Chemical mediators involved: Histamine, prostaglandins, platelet-activating factor, kinins, and nitric oxide (NO).
- Increased permeability of the microvasculature: It leads to the escape of protein-rich fluid from circulation into the extravascular tissues.
- Chemical mediators involved: Histamine, leukotrienes, platelet-activating factor, and kinins.

- Slowing of blood flow: Vasodilatation and loss of fluid leads to the concentration of RBCs in small vessels and increased viscosity of the blood. These changes result in the engorgement of small vessels with slowly moving red cells, a condition termed stasis.
- Stasis: It is responsible for vascular congestion and localized redness of the involved tissue.
- Leukocyte events: As stasis develops, blood leukocytes (mainly neutrophils), accumulate along the vascular endothelium.
Increased Vascular Permeability (Vascular Leakage):
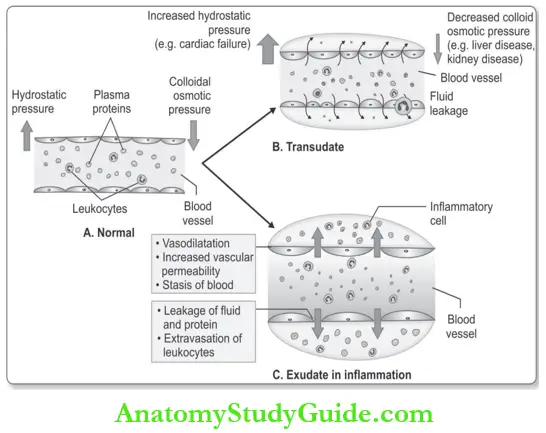
- Exudation: It is defined as the process of escape of fluid, proteins, and circulating blood cells from the vessels into the interstitial tissue or body cavities.
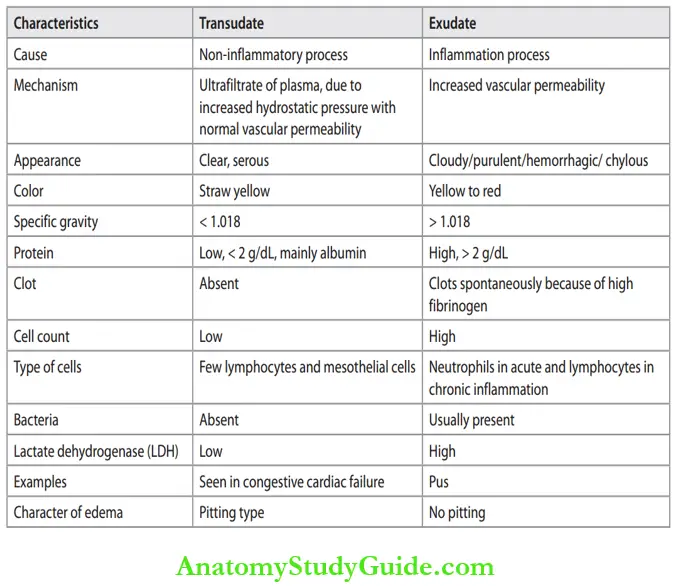
- The escape of a protein-rich fluid causes edema and is one of the cardinal signs of inflammation. Differences between transudate and exudate are listed in Table.
Mechanism of Increased Vascular Permeability:
Describe the mechanism of increased vascular permeability
Several mechanisms can cause increased vascular permeability of postcapillary venules:
- Contraction of endothelial cells: It produces increased inter endothelial spaces and results in edema.
- A most common mechanism of vascular leakage.
- Occurs immediately after injury and is usually short-lived (15–30 minutes) and hence called as immediate transient response.
- In some mild injuries (for example, After burns, irradiation or ultraviolet radiation, and certain bacterial toxins), vascular leakage develops after a delay of 2 to 12 hours and it lasts for many hours or even days.
- This delayed prolonged leakage may be due to the contraction of endothelial cells or mild endothelial damage (for example, Sunburn)
- Chemical mediators involved: Histamine, bradykinin, leukotrienes, and the neuropeptide substance P.
- Direct endothelial injury: It causes necrosis and detachment of endothelial cells, for example, burns, or infection by microbes. In most cases, leakage starts immediately after injury and is sustained for several hours until the damaged vessels are thrombosed or repaired. Hence, it is called as immediate sustained response.
- Leukocyte-mediated vascular injury: Th leukocyte (mainly neutrophils) which adhere to the endothelium during inflammation may injure the endothelial cells.
- Increased transcytosis: The process of transport of fluids and proteins through the endothelial cell (channels called vesiculovacuolar organelle) is known as transcytosis. However, their role in the vascular permeability of acute inflammation is uncertain.
- Leakage from new blood vessels: During repair new blood vessels are formed (angiogenesis). These vessels are leaky till the endothelial cells mature.
Difference between Transudate and exudate:
Responses of Lymphatic Vessels and Lymph Nodes:
- Apart from blood vessels, lymphatic vessels also participate in acute inflammation. Lymphatic vessels normally drain the small quantity of extravascular fluid that has escaped out of capillaries.
- Increased vascular permeability in inflammation produces an accumulation of fluid in the extravascular space (i.e. edema). In inflammation, there is an increased flow of lymph that helps to drain this edema fluid.
- In addition to fluid, leukocytes, cell debris, and microbes, may also flow into lymph way along with lymph.
- Secondary inflammation may occur in the draining lymphatics (lymphangitis characterized by the presence of red streaks along the course of the lymphatic channels draining a skin wound) and also in the draining lymph nodes (lymphadenitis). Inflamed draining lymph nodes are often painful and enlarged.
- These lymph nodes are termed reactive, or inflammatory, lymphadenitis.
Leukocytic/Cellular Events
Describe leukocyte/cellular events in acute inflammation.
This process delivers leukocytes capable of phagocytosis (neutrophils and macrophages) to the site of injury. The events can be divided into: Leukocyte recruitment Phagocytosis and clearance of the injurious agent.
1. Leukocyte Recruitment/Extravasation:
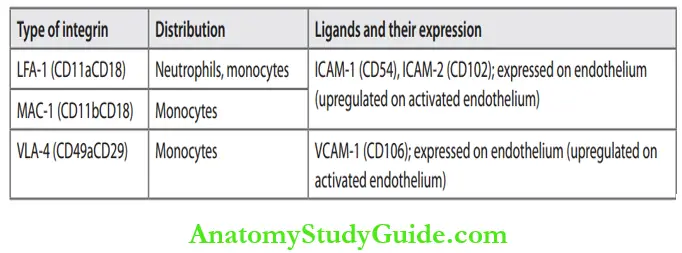
Normally, leukocytes move rapidly in the blood, and during inflammation, they slow down and escape to the site of injury/causative agent in the extravascular space. Leukocyte extravasation is the process of migration of leukocytes from the lumen of the vessel to the site of injury in the extravascular tissues. It is a multi-step process that is mediated and controlled by adhesion molecules and cytokines called chemokines.
Steps in Leukocyte Recruitment/Extravasation:
- In the Vascular Lumen:
- Margination: When the blood flow slows down (stasis), leukocytes (mainly neutrophils)
move towards the peripheral column and accumulate along on the endothelial surface of
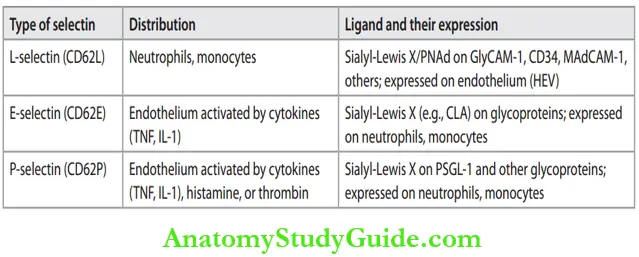
vessels. This process of redistribution of leukocytes is termed margination. - Rolling: Marginated leukocytes attach weakly to the endothelium, detach and bind again with a mild jumping movement. It causes the rolling of leukocytes along the endothelial surface.
- Molecules involved: Selectin family of adhesive molecules and its complementary ligands
- Margination: When the blood flow slows down (stasis), leukocytes (mainly neutrophils)

-
- Chemical mediators involved: Cytokines such as:
- Tumor necrosis factor (TNF)
- Interleukin-1 (IL-1) and chemokines (chemoattractant cytokines).
- Chemical mediators involved: Cytokines such as:
- Adhesion of leukocyte to endothelium: Endothelium gets activated and leukocytes bind more firmly.
- Molecules involved: Th adhesion/attachment of leukocytes to endothelial cells is mediated by complementary adhesion molecules on these two cell types. The expression of adhesive molecules is enhanced by cytokines.
- Chemical mediators involved:
- Endothelial cells are activated by cytokines namely: TNF and IL-1 and increase the expression of two ligands for integrins on leukocytes.
- Chemokines are chemoattractant cytokines that cause leukocyte activation and conversion of low-affinity integrins on leukocytes to the high-affinity state resulting in firm adhesion of the leukocytes to the endothelium.
Leukocyte Migration through Vessel Wall:
- Leukocyte migration through endothelium: Migration of the leukocytes through the endothelium is called transmigration or diapedesis. Leukocytes migrate through the vessel wall by squeezing through the intercellular junctions between the endothelial cells.
- Molecules involved: CD31 (platelet endothelial cell adhesion molecule [PECAM-1]) expressed on leukocytes and endothelial cells and ICAM-1. It occurs mainly in postcapillary venules.
- Migration across the basement membrane: Leukocytes penetrate the basement membrane of the vessel by secreting collagenases.
Outside the Vessel Wall:
After penetration of the basement membrane of a vessel, leukocytes move in the tissues toward the site of injury by a process called chemotaxis. It can be simply defied as the locomotion of leukocytes along a chemical gradient.
Chemotaxis of leukocytes Define and write short notes on chemotaxis.
Definition of leukocytes: Chemotaxis is defined as the process of migration of leukocytes toward the inflammatory stimulus in the direction of the gradient of locally produced chemoattractants.
Chemoattractants:
- Exogenous: Bacterial products (for example, N-formylmethionine terminal amino acid)
- Endogenous:
- Cytokines, mainly the chemokine family (for example, IL-8)
- Complement components: C5a, C3a
- Arachidonic acid metabolites of lipoxygenase pathway: Leukotriene B4 (LTB4).
Accumulation of leukocytes at the sites of infection and injury:
Achieved by binding of leukocytes to the extracellular matrix proteins through integrins and CD44.
Type of leukocyte infiltrates:
- Neutrophils: Predominantly during the first 6 to 24 hours.
- Monocytes: Neutrophils are replaced by monocytes in 24 to 48 hours.
2. Phagocytosis and Clearance of the Offending Agent:
Develops in two sequential events:
- Recognition of microbes, necrotic cells and foreign substances: Leukocytes recognize microbes, necrotic cells, and foreign substances by cell surface receptors known as “pattern recognition receptors”.
- Activation of leukocytes: Recognition of microbes or dead cells by the receptors initiates several responses in leukocytes together known as leukocyte activation.
- The most important functional responses of leukocyte activation is phagocytosis and intracellular killing.
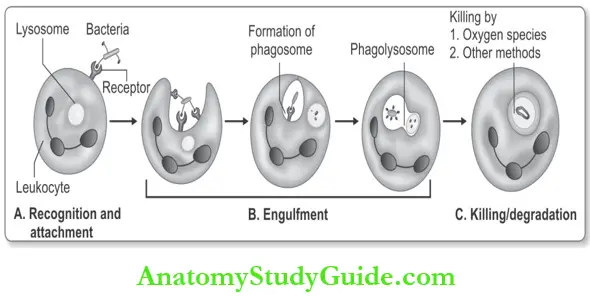
Phagocytosis or Write a short note on phagocytosis.
Many leukocytes recognize, internalize, and digest foreign materials, microorganisms, or cellular debris by a process termed phagocytosis.
It consists of three steps:
- Recognition and attachment
- Engulfment
- Killing or degradation of the ingested material.
Difference options and their corresponding on leukocyte:

1. Recognition and Attachment:
- Receptors on the surface of phagocytic cells recognize components of microbes and necrotic cells. Leukocytes express several receptors that recognize external stimuli. These include
- Receptors for microbial products ( for example, Toll-like receptors—TLRs),
- G protein–coupled receptors (recognize N-formyl methionine residues), 3) receptors for cytokines (for INF-γ), and 4) receptors for opsonins (described below).
- Receptors for opsonins (phagocytic receptor): Th phagocytosis is enhanced when leukocyte receptors recognize microbes coated by specific host proteins known as opsonins.
- The major opsonins are IgG antibodies, the C3b breakdown product of complement, and certain plasma lectins called collectins.
2. Engulfment:
The next step in phagocytosis is the engulfment and formation of a phagocytic vacuole. Phagocytosis is dependent on the polymerization of actin filaments.
- Phagosome: Extensions of the cytoplasm of leukocyte form pseudopods surrounding the particle to be ingested and forms a vesicle or vacuole called a phagosome.
- Phagolysosome: Th membrane of the phagosome fuses with the membrane of lysosome to form a phagolysosome. Lysosomal granules are discharged into this phagolysosome.
3. Killing and Degradation: Killing and degradation of ingested microbial agents/particles occurs within neutrophils and macrophages.
The most important microbicidal agents are:
- Reactive oxygen species and
- Lysosomal enzymes.
1. Reactive Oxygen Species (ROS):
Types of ROS are:
- Superoxide anion (O2•one electron) – Weak
- Hydrogen peroxide (H2O2, two electrons) – Weak
- Hydroxyl ions (•OH), three electrons – Highly reactive.
Mechanism of production: In the phagocytic vacuole of leukocyte, rapid activation of NADPH oxidase (also called phagocyte oxidase), oxidizes NADPH (reduced nicotinamide-adenine dinucleotide phosphate) to NADP. During the process, oxygen is reduced to superoxide anion (O2•).
- O2• is converted into hydrogen peroxide (H2O2) by spontaneous dismutation → O2•+ 2H → H2O2
- The amount of H2O2 is insufficient to kill most of the microbes by itself but the enzyme myeloperoxidase (MPO) present in the azurophilic granules of neutrophils can convert it into a powerful ROS.
- MPO in the presence of a halide such as Cl–, converts H2O2 to hypochlorous radical (HOCl•), which is a potent oxidant and antimicrobial agent.
Hypochlorite (HOCl•) destroys microbes either by halogenation or by proteins and lipid peroxidation. H2O2 is also converted to hydroxyl radical (•OH) which is also a powerful destructive agent.
Reactive nitrogen species: NO, which is generated from arginine by the action of nitric oxide synthase (NOS), can kill microbes similar to ROS.
- NO reacts with superoxide (O2• ) and produces highly reactive free radical peroxynitrite (ONOO•).
2. Lysosomal Enzymes:
Acid hydrolases of lysosomes degrade the dead microorganisms. Elastase can kill bacteria.
Constituents of leukocyte granules: Th microbicidal substances within leukocyte cytoplasmic granules include:
- Bactericidal permeability-increasing protein
- Lysozyme and lactoferrin
The major basic protein (MBP) present in eosinophils is cytotoxic to many parasites:
- Defensins are toxic to microbes
- Cathelicidins: These are anti-microbial proteins in neutrophils and other cells. They are very effective against Mycobacterium tuberculosis.
Chemical Mediators Of Inflammation
Write a short note on the role of different mediators in different reactions of inflammation.
Definition of inflammation: Substances that initiate and regulate inflammatory reactions are called as mediators of inflammation. Numerous chemical mediators are responsible for inflammatory reactions.
List chemical mediators of inflammation. Role of chemical mediators in inflammation. Name the cell-derived mediators of inflammation. Name the plasma-derived mediators of inflammation.
General Features of Mediators:
- Source of mediators: Mediators are derived either from cells or from plasma proteins
- Cell-derived mediators:
- Present either as preformed molecules (for example, Histamine in mast cell granules) or are synthesized de novo (for example, Prostaglandins, Cytokines) in response to a stimulus.
- Produced usually by platelets, neutrophils, monocytes/macrophages, and mast cells.
- Plasma-derived mediators: Produced mainly in the liver and present in the circulation as inactive precursors, which require activation (for example, Complement proteins, kinins).
- Cell-derived mediators:
- Tightly regulated actions:
- Inter-related: One mediator can stimulate the release of other mediators. The secondary mediators may have similar, different, or even opposite actions.
- Most act by binding to specific receptors on target cells.
- Diverse targets: Target cell type varies depending on the type of mediator. They can act on one or a few or many diverse targets or may have different effects on different types of cells.
- Short-lived: Most of these mediators have a short-lifespan.
The main mediators involved in the inflammatory reaction are listed in Table.
Main chemical mediators of acute inflammation:

Cell-Derived Mediators
Vasoactive Amines: Histamine and Serotonin:
Histamine and serotonin are the first mediators to be released during inflammation, which are stored as preformed molecules in cells.
1. Histamine: It is a preformed vasoactive mediator. Responsible for an immediate transient response.
- Source: Mast cells (richest source), blood basophils, and platelets.
- Stimuli:
- Physical injury (for example, Trauma, cold, heat)
- Immune reactions in which antibodies bind to mast cells ( (for example, Allergic reactions)
- Other chemical mediators: C3a and C5a, leukocyte-derived histamine-releasing proteins, neuropeptides (for example, Substance P), and Cytokines (IL-1, IL-8).
- Actions:
- Dilation of arterioles and
- Increase of vascular permeability.
2. Serotonin (5-hydroxytryptamine): It is a preformed vasoactive mediator.
- Source: Platelets, some neurons, and enterochromaffin cells in the gastrointestinal tract.
- Stimulus: Platelet aggregation and antigen-antibody complexes.
- Actions: Similar to those of histamine.
Arachidonic Acid (AA) Metabolites (Prostaglandins, Leukotrienes, and Lipoxins):
Arachidonic Acid (AA):
- Source: Derived from cell membrane phospholipids mainly by the enzyme phospholipase A2.
- Stimuli: Mechanical, chemical, and physical stimuli or other mediators (for example, C5a).
- AA metabolism: Occurs along two major enzymatic pathways. These are the cyclooxygenase pathway (produces prostaglandins) and the lipoxygenase pathway (produces leukotrienes and lipoxins).
1. Products of cyclooxygenase pathway:
Products: Most important in inflammation are PGE2, PGD2, PGI2 (prostacyclin), and TxA2 (thromboxane A2).
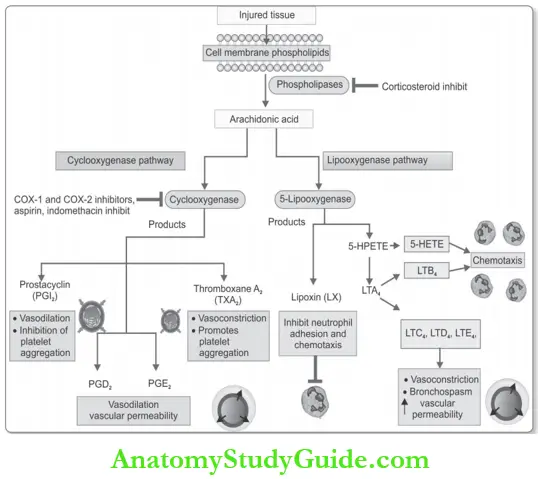
- Mechanism: Thy are produced from AA by the actions of two cyclooxygenases, COX-1 and COX-2.
- Local effects:
- TxA2: Vasoconstriction and promotes platelet-aggregation
- Prostacyclin (PGI2): Vasodilator and inhibits platelet aggregation
- PGD2 and PGE2: Vasodilation and increased permeability. PGD2 is also a chemoattractant for neutrophils.
- Systemic effects:
- Prostaglandins are responsible for pain and fever in inflammation.
- PGE2 causes cytokine-induced fever during infections.
2. Products of lipoxygenase pathway:
- Leukotrienes and
- Lipoxins.
Leukotrienes: Products and their actions:
- 5-hydroxyeicosatetraenoic acid (5-HETE): Chemotactic for neutrophils, and is the precursor of the leukotrienes.
- LTB4
- Chemotactic agent
- Neutrophil activation causes adhesion to endothelium, generation of ROS, and
release of lysosomal enzymes.
- Leukotrienes C4, D4, and E4(LTC4, LTD4, LTE4):
- Vasoconstriction
- Bronchospasm (in asthma)
- Increased vascular permeability.
Lipoxins (LXs):
- Actions: Inhibit inflammation
- Inhibit neutrophil chemotaxis and recruitment.
- Inhibit leukocyte adhesion to endothelium.
The main actions of arachidonic acid metabolites (eicosanoids) involved in inflammation are presented in Table.
Main actions of arachidonic acid metabolites (eicosanoids) in inflammation:

Platelet-activating Factor (PAF):
- Action: Multiple inflammatory effects:
- Vascular reactions: Vasodilation and increased vascular permeability.
- Cellular reactions: Increased leukocyte adhesion to endothelium, chemotaxis.
- Others: Increases the synthesis of other mediators, mainly eicosanoids.
Reactive Oxygen Species (ROS):
ROS are chemically reactive oxygen-derived free radicals. Normally, they are rapidly inactivated. But increased production can cause cell injury.
- Cell of origin: Leukocytes (neutrophils and macrophages).
- Mechanism of production: Leukocytes during phagocytosis (after exposure to microbes, chemokines, and immune complexes) generate oxygen-derived free radicals
Types of production:
- Superoxide anion (O2•)
- Hydrogen peroxide (H2O2)
- and Hydroxyl radical (•OH)
- O2• can combine with NO to form reactive nitrogen species (peroxynitrite ONOO–)
Actions of production:
- Physiologic Function: ROS in leukocytes destroys phagocytosed microbes and necrotic cells.
- Pathological actions:
- Endothelial cell damage, which causes increased vascular permeability
- Injury to other cells: e.g. tumor cells, parenchymal cells, and red blood cells
- Inactivation of antiproteases, such as α1-antitrypsin, for example, Destruction of elastic tissues in emphysema of the lung.
Nitric Oxide (NO):
- NO is a soluble, free-radical gas that causes vasodilation (known as endothelium-derived relaxing factor).
- Source: Many cells, such as endothelial cells, macrophages, and neurons in the brain.
- Synthesis: Synthesized from l-arginine, molecular oxygen, and NADPH by the enzyme nitric oxide synthase (eNOS).
Types of Nitric Oxide:
Three isoforms of NOS:
- Type 1 neuronal (nNOS)
- Type 2 inducible (iNOS), and
- Type 3 endothelial (NOS).
Action: It acts in a paracrine manner on target cells:
- Vasodilatation by relaxing vascular smooth muscle cells.
- Controls inflmmatory responses by inhibiting leukocyte recruitment and adhesion.
- Reduced platelet adhesion, aggregation and degranulation.
- Microbicidal activity.
Cytokines and Chemokines:
These are polypeptides that function as mediators in immune responses and in inflammation (acute and chronic).
- Source: Cytokines are secreted by many types of cells (activated lymphocytes and macrophages, endothelial, epithelial, and connective tissue cells).
Tumor Necrosis Factor and Interleukin-1:
These are the two major cytokines involved in inflammation:
- Source: Activated macrophages.
- Stimuli: Endotoxin and other microbial products, immune complexes, physical injury, and many inflammatory stimuli.
Actions in inflammation:
- Local effects:
- Endothelial activation: Endothelial activation and increased expression of endothelial adhesion molecules.
- Activation of leukocytes and other cells: TNF increases the responses of neutrophils to other stimuli (for example, Bacterial endotoxin).
- During repair: Proliferation of fibroblasts and increased synthesis of collagen.
- Systemic effects:
- Fever
- Leukocytosis
- Systemic acute-phase reactions
- Suppresses appetite: TNF contributes to cachexia seen in some chronic infections.
Chemokines
Chemotactic cytokines or chemokines are small proteins, which selectively attract various leukocytes to the site of inflammation.

Classification: Chemokines are classified into four major groups namely:
- C-X-C chemokines
- C-C chemokines
- C chemokines and
- C-X-3C chemokines.
Action: Chemotaxis of monocytes, eosinophils, basophils, and lymphocytes except neutrophils. They activate leukocytes and promote their recruitment to the sites of inflammation.
Other Cytokines in Acute Inflammation:
- IL-6, produced by macrophages and other cells is involved in local and systemic reactions.
- IL-17 produced by T lymphocytes promotes neutrophil recruitment.
Lysosomal Constituents of Leukocytes:
1. Neutrophils
Types of granules:
- Smaller species (or secondary) granules: They contain lysozyme, collagenase, gelatinase, lactoferrin, plasminogen activator, histaminase, and alkaline phosphatase.
- Larger azurophil (or primary) granules: Thy contain myeloperoxidase, bactericidal factors (lysozyme, defensins), acid hydrolases, and a variety of neutral proteases (elastase, cathepsin G, nonspecific collagenases, proteinase).
Monocytes and Macrophages:
They also contain acid hydrolases, collagenase, elastase, phospholipase, and plasminogen activator. These are active mainly in chronic inflmmation.
2. Neuropeptides:
- These are small peptides, such as substance P and neurokinin A.
- Source: Secreted by sensory nerves and various leukocytes.
- Action: Vasodilation and increased vascular permeability.
Plasma-Derived Mediators
Chemical mediators derived from plasma proteins belong to three interrelated systems:
- Complement
- Kinin
- Clotting systems.
1. Complement System:
What are the three methods of complement activation and their effector function in acute inflammation?
The complement system is a group of plasma proteins synthesized in the liver, and are numbered C1 to C9.
Pathways of complement system activation: The decisive step in complement
activation is the proteolysis of the third component, C3.
Cleavage of C3 can occur by any one of three pathways:
- Classical pathway: It is activated by antigen-antibody (Ag-Ab) complexes.
- Alternative pathway: It is triggered by microbial surface molecules (for example, Endotoxin, or LPS), complex polysaccharides, cobra venom, and other substances, in the absence of antibodies.
- Lectin pathway: It directly activates C1 when plasma mannose-binding lectin binds to mannose on microbes.’

Functions of Complement:
- Anti-infective Functions:
- Leukocyte activation, adhesion, and chemotaxis: C5a causes leukocyte activation, and adhesion, and C3a and C5a are powerful chemotactic agents for neutrophils, monocytes, eosinophils, and basophils.
- Opsonization and promote phagocytosis: C3b and its cleavage product iC3b (inactive C3b) act as opsonins and promote phagocytosis by neutrophils and macrophages through
surface receptors for these complement fragments. - Cell and bacterial lysis: Th deposition of the MAC (C5b-C9) on cells creates pores, which allow water and ions to enter into the cells and result in death (lysis) of the cells and bacteria.
- Increased vascular permeability: C3a, C5a complement components stimulate histamine release from mast cells and thus increase vascular permeability and cause vasodilation. They are called anaphylatoxins because their actions are similar to mast cell mediators involved in anaphylaxis.
- Activation of AA: C5a activates the lipoxygenase pathway of AA metabolism in neutrophils and monocytes, thereby causing the release of more chemical mediators.
The interplay between innate and adaptive immune system:
- Defense against microbes through innate and adaptive immunity
Other Functions:
- Clearance of:
- Immune complexes (Clq, C3)
- Apoptotic cells (Clq, C3).
Coagulation and Kinin Systems:
Inflammation and the clotting system are intertwined with each other. Activated Hageman factor (factor XIIa) activates the four systems involved in the inflammatory response.
- Activation of the fibrinolytic system: Factor XIIa stimulates the fibrinolytic system by converting plasminogen to plasmin. The role of the fibrinolytic system in inflammation are:
- Activation of the complement system.
- Fibrin split products: Plasmin degrades fibrin to form fibrin split products, which may increase vascular permeability.
- Activation of the Kinin system:
- Activation of the alternative complement pathway: Factor XIIa can activate the alternate complement pathway.
- Activation of the coagulation system: Factor XIIa activates the coagulation system and forms thrombin, which has inflammatory properties.
Kinins System:
Kinins are vasoactive peptides derived from plasma proteins.
- Mechanism of production: Factor XIIa converts prekallikrein to kallikrein, which in turn cleaves high-molecular-weight kininogen to produce bradykinin.
 ‘
‘
Important mediators involved in acute inflammation:

- Actions of bradykinin:
- Increases vascular permeability
- Pain when injected into the skin.
- Actions of kallikrein:
- Potent activator of Hageman factor
- Chemotactic activity: Directly converts C5 to the chemoattractant product C5a.
The most important mediators involved in acute inflammation are summarized in Table.
Outcomes Of Acute Inflammation
Write a short note on the outcomes (fate) of acute inflammation.
- Resolution: Complete return of tissue architecture to normal following acute inflammation. It occurs:
- When the injury is limited or short-lived
- With no or minimal tissue damage
- When injured tissue is capable of regeneration.
- Organization/healing by fibrosis: The process of replacement of dead tissue by living tissue, which matures to form scar tissue is known as organization. It occurs:
- When there is plenty of fibrin exudation in tissue or serous cavities (pleura, peritoneum) which cannot be removed or cleared.
- In the presence of significant tissue destruction.
- With inflammation in tissues incapable of regeneration.
- This process involves the growing of connective tissue into the area of tissue damage or exudate and is converted into a mass of fibrous tissue (scar).
- Abscess: Localized collection of pus is called an abscess. If the area of acute inflmmation is walled of by inflammatory cells and fibrosis, neutrophil products destroy the tissue and form an abscess.

Progression to chronic inflammation: Chronic inflammation may follow acute inflammation, or it may be chronic from the beginning itself. Acute progress to chronic when the acute inflammatory response cannot be resolved. This may be due to:
- Persistence of the injurious agent or
- Abnormality in the process of healing.
- Examples:
- Bacterial infection of the lung may begin as acute inflammation (pneumonia). But when it fails to resolve, it can cause extensive tissue destruction and form a cavity with chronic inflammation known as a lung abscess.
- Acute osteomyelitis if not treated properly may progress to chronic osteomyelitis.
- Chronic inflammation with a persisting stimulus results in peptic ulcer of the duodenum or stomach, which may persist for months or years.
Morphological Types/Patterns Of Acute Inflammation
Write a short note on morphological types/patterns of acute inflammatory reaction with suitable examples.
Gross and microscopic appearances can often provide clues about the cause:
Serous Inflammation:
- Characterized by the marked outpouring of a thin serous fluid.
- Serous exudate or effusion is yellow, straw-like in color, and microscopically shows either few or no cells.
- Example:
- Skin blister formed in burn or viral infection.
- Inflmmation of synovium (synovitis).
- Pleural effusion as a complication of lobar pneumonia.
Fibrinous Inflammation:
- A marked increase in vascular permeability leads to the escape of large molecules like fibrinogen from the lumen of the vessel into the extravascular space and forms fibrin. The exudate is rich in firing is called a fibrinous exudate.
- A fibrinous exudate is mostly observed with inflammation in the lining of body cavities, such as the meninges, pericardium, and pleura. When a fibrinous exudate develops on a 60 serosal surface, such as the pleura or pericardium, it is known as fibrinous pleuritis or fibrinous pericarditis.
- Microscopically, firin’ appears as an eosinophilic or pink meshwork of threads or pink amorphous coagulum.

- For example, fibrinous pericarditis is seen in rheumatic fever and is classically known as “bread and butter” pericarditis.
Suppurative or Purulent Inflammation: Abscess
- It is characterized by the production of large amounts of pus or purulent exudate.
- Microscopically: Shows neutrophils, liquefactive necrosis, and edema fluid. Bacteria (for example, Staphylococci) that produce localized suppuration are called pyogenic (pus-producing) bacteria. For example acute appendicitis.
- Abscesses: It is the localized collections of purulent inflammatory exudates in a tissue, an organ, or a confined space. Abscesses have a central necrotic focus (consisting of necrotic leukocytes and necrotic parenchymal cells) surrounded by a zone of preserved neutrophils.
- If pus accumulates in hollow organs or pleural cavities, it is known as empyema e.g. Boil caused by Staphylococcus aureus.
Hemorrhagic Inflammation:
When inflammation is associated with severe vascular injury or deficiency of coagulation factors, it causes hemorrhagic inflammation, for example, Acute pancreatitis due to proteolytic destruction of vascular walls.
Catarrhal Inflammation:
Acute inflammation of a mucous membrane is accompanied by excessive secretion of mucus and the appearance is described as catarrhal, for example, Common cold.
Membranous Inflammation:
In this type, the epithelium is covered by a membrane consisting of firin’, desquamated epithelial cells, and inflammatory cells, for example, Pharyngitis or laryngitis due to Corynebacterium diphtheria.
Pseudomembranous Inflammation:
Superficial mucosal ulceration covered by sloughed mucosa, fibrin, mucus and inflammatory cells. For example, pseudomembranous colitis due to Clostridium’s difficult colonization of the bowel, usually following broad-spectrum antibiotic treatment.
Necrotizing (Gangrenous) Inflammation:
The combination of necrosis and bacterial putrefaction is gangrene, for example, Gangrenous appendicitis.
Ulcer:
An ulcer is defined as a local defect, or excavation, of the surface of an organ or tissue.
Common sites:
- Mucosa of the mouth, stomach (for example, Peptic ulcer of the stomach or duodenum intestines, or genitourinary tract.
- Skin and subcutaneous tissue of the lower extremities ((for example, Varicose ulcers).
Systemic Effects Of Inflammation
Write a short note on the systemic effects of inflammation.
Systemic changes in acute inflammation are collectively known as an acute-phase response, or systemic inflammatory response syndrome (SIRS).
Causes of inflammation:
- Due to cytokines produced by leukocytes, in response to infections or immune reactions.
- The most important cytokines are TNF, IL-1, and IL-6.
The clinical and pathologic changes of acute-phase response are:
1. Fever:
- Pyrogens: These are molecules that cause fever. It may be exogenous (bacterial products, like LPS), which stimulate leukocytes to release endogenous pyrogens (cytokines such as IL-1 and TNF). The cytokines increase the enzymes cyclooxygenases resulting in the conversion of AA into prostaglandins.
- Pyrogens and prostaglandins may act on the hypothalamic thermoregulatory center causing fever.
2. Raised plasma levels of acute-phase proteins: These are plasma proteins synthesized in the liver and may be markedly raised in response to inflammatory stimuli.
Types of acute-phase proteins:
- C-reactive protein (CRP)
- Fibrinogen
- Serum amyloid A (SAA) protein.
- Their synthesis by hepatocytes is increased by cytokines, especially IL-6 (for CRP and fibrinogen) and IL-1 or TNF (for SAA).
- Actions/functions:
- Many acute-phase proteins (CRP and SAA) bind to microbial cell walls and may act as opsonins.
- Fibrinogen binds to red cells to form stacks (rouleaux) and is responsible for raised erythrocyte sedimentation rate (ESR).
- During acute inflammation, acute-phase proteins have beneficial effects but prolonged production (especially SAA) like in chronic inflammation causes secondary amyloidosis.
3. Changes in the leukocytes:
- Leukocytosis: Total leukocyte count of more than 11,000/μL is termed as leukocytosis. Common in inflammatory reactions, especially those caused by bacterial infections.
- Count: May be increased up to 15,000 or 20,000 cells/μL. Sometimes, it may be extremely high reaching 40,000 to 100,000/μL associated with more immature neutrophils in the blood (shift to the left) and are called as leukemoid reactions, similar to the white cell counts found in leukemia. It is important to distinguish it from leukemia which is a malignant disease.
- Cause: It is due to the increased release of leukocytes from the bone marrow caused by cytokines, including Colony stimulating factors (CSFs), TNF, and IL-1.
- Bacterial infections cause an increase in the blood neutrophil count known as
neutrophilia.
- Lymphocytosis: It is seen in viral infections (e.g. Infectious mononucleosis, mumps,
and German measles). - Eosinophilia: It is seen in bronchial asthma, allergy, and parasitic infestations.
- Leukopenia: A decreased number of circulating white cells is associated with a few
infections like typhoid fever and some viruses, rickettsia, and certain protozoa.
4. Other features of the acute-phase response: Includes:
- Increased pulse and blood pressure.
- Anorexia and malaise, are probably due to cytokines acting on brain cells.
- In severe bacterial infections (sepsis), cytokines (mainly TNF and IL-1) may be produced in large quantities and can result in disseminated intravascular coagulation and cardiovascular failure.
Chronic Inflammation
Definition of Chronic: Chronic inflammation is defined as inflammation of prolonged duration (weeks or months) in which inflammation, tissue damage, and healing occur at the same time, in varying combinations.
Chronic inflammation may:
- Follow an acute inflammation, which does not resolve (for example,Chronic osteomyelitis) or
- Begin as an insidious, low-grade, chronic, response without any acute inflammatory reaction.
Causes of Chronic Inflammation:
What are the causes of chronic inflammation?
1. Persistent infections: Microbes that are difficult to eradicate elicit delayed-type of hypersensitivity and produce chronic inflammation, for example,Mycobacteria, and certain viruses, fungi, and parasites. Some agents may cause a distinct pattern of chronic inflammation known as a granulomatous reaction.
2. Immune-mediated inflammatory diseases:
- Autoimmune diseases: For example Rheumatoid arthritis.
- Allergic reactions: For example Bronchial asthma.
- Unregulated immune response: For example Inflammatory bowel disease.
3. Prolonged exposure to toxic injurious agents
- Exogenous: Silica is a nondegradable inanimate exogenous material. If persons are exposed to silica particles for long time, it causes an inflammatory lung disease called
silicosis. - Endogenous: Atherosclerosis is a disease of arterial intima, probably represents a
chronic inflammatory process partly due to endogenous toxic plasma lipid components.
Morphologic Features of chronic inflammation:
Chronic inflammation is characterized by:
- Mononuclear cells infiltrate Macrophages, lymphocytes, and plasma cells.
- Tissue destruction: Due to the persistence of causative agents or by the inflammatory cells.
- Healing by fibrosis.
Chronic Inflammatory Cells And Mediators
Write a short note on cells of chronic inflammation.
Macrophages of chronic inflammation:
Macrophage is the predominant cell in chronic inflammation.
Macrophage Events in Inflammation Or Mention the role of macrophages in chronic inflammation.
- Monocytes also emigrate into extravascular tissues early in acute inflammation, and within 48 hours, they are the predominant cell type.
- On reaching extravascular tissue, the monocyte is transformed into a larger phagocytic cell known as a tissue macrophage.
Macrophage Activation of Chronic Inflammation:
Tissue macrophages are activated by two major pathways:
Classical of chronic inflammation:
- Mediators of activation: It is brought out mainly by
- Microbial products: For example, Endotoxin
- T cell-derived signals: Mainly cytokines ( For example, IFN-γ)
- Foreign substances: For example, Crystals and particulate matter
- Products of activated macrophages:
- Lysosomal enzymes
- Nitric oxide
- Reactive oxygen species (ROS)
- Function: Phagocytosis and killing/elimination of ingested microbes.
Alternate of chronic inflammation:
- Mediators of activation: It is brought out mainly by cytokines IL-4 and IL-13 produced
by T-cells and other cells. - Function: Initiation of the tissue repair, (they are not bactericidal).
Functions of chronic inflammation:
- Phagocytosis: Ingestion and elimination of microbes and necrotic tissue.
- Initiation of the tissue repair.
- Secretion of mediators of inflammation: These include cytokines (TNF, IL-1, chemokines etc.) and arachidonic acid metabolites.
- Display signal to T-cells and respond to signals from T-cells: Ths is responsible for the
feedback loop for defense against many microbes by the cell-mediated immune response.
Lymphocytes of chronic inflammation:
- B and T-lymphocyte: They are found in both antibody-mediated and cell-mediated
immune reactions. - B lymphocytes: Thy may develop into plasma cells and produce antibodies either against
foreign or self-antigens in the inflammatory site. - T lymphocytes: Important being CD4+ helper T cells which have 3 subtypes namely:
- TH1: Produce INF-ϒ and activates macrophage in the classical pathway.
- TH2: Produce IL-4, IL-5, and IL-13 which recruit and activate eosinophils and activate macrophages through alternate pathways. Involved in defense against helminthic infestation and allergic reactions.
- T H17: Produce IL-17 and other cytokines which recruit neutrophils and monocytes.
Other Cells of chronic inflammation:
- Plasma cells: They are derived from activated B lymphocytes and produce antibodies either against foreign or self-antigens.
- Eosinophils: They are seen in immune reactions mediated by IgE and in parasitic infections. A chemokine, which attracts eosinophil recruitment is eotaxin. Eosinophils granules contain a major basic protein that is toxic to parasites and also destroys the epithelial cells.
- Mast cells: They are distributed in connective tissues and participate in both acute and chronic inflammatory reactions. They are seen in allergic reactions to foods, insect venom, or drugs.
Granulomatous Inflammation
Define and classify granuloma.
Definition of Granuloma: A granuloma is defined as a distinctive type of chronic inflammation characterized by microscopic aggregation of activated macrophages (that are transformed into epithelium-like/epithelioid cells) with scattered lymphocytes. Older granulomas in addition show the rim of fibroblasts and connective tissue as the outermost layer.
- Epithelioid cells: These are modifid macrophages that resemble epithelial cells.
- They have a pale pink granular cytoplasm with indistinct cell borders, often appearing to merge into one another.
- The nucleus is oval or elongated and may show the folding of the nuclear membrane. The nucleus is less dense than that of a lymphocyte.
- They have a pale pink granular cytoplasm with indistinct cell borders, often appearing to merge into one another.
- Giant cells: Epithelioid cells frequently fuse to form giant cells and are found in the periphery or sometimes in the center of granulomas.
- These giant cells may attain diameters of 40 to 50 μm and have many small nuclei.
- Nuclei may be as many as 20 or more which are and may be arranged either peripherally (Langhans-type giant cell) or haphazardly (foreign body–type giant cell).
Cause of Granuloma Formation:
Give examples of chronic granulomatous inflammation.
1. Persistent T-cell response against certain microbes: For example, Mycobacterium tuberculosis.
Granuloma in tuberculosis is referred to as a tubercle and usually shows central caseous necrosis. Caseous necrosis is seen in tuberculosis and is rare in other
granulomatous diseases. Sometimes, it may be necessary to perform additional tests/
investigations to identify the etiologic agent.
- Special stains, for example, Acid-fast stains for tubercle bacilli
- Culture methods, for example, Tuberculosis and fungal diseases
- Molecular techniques (for example, Polymerase chain reaction in tuberculosis)
- Serologic studies (for example, Syphilis).
Examples of granulomatous inflammation:
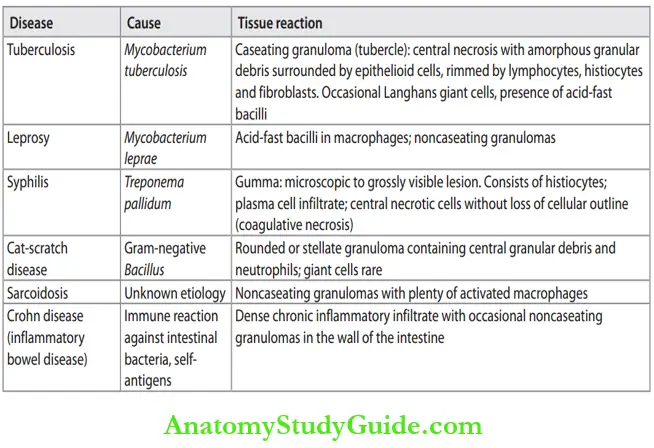

2. Immune-mediated inflammatory reaction: For example, Crohn’s disease.
3. Foreign body granuloma: These are produced by relatively inert foreign bodies. For example, foreign body granulomas form around materials such as talc (associated with intravenous drug abuse), sutures, or other fibers. Epithelioid cells and giant cells are opposed to the surface of the foreign body. Sometimes etiology is not known.
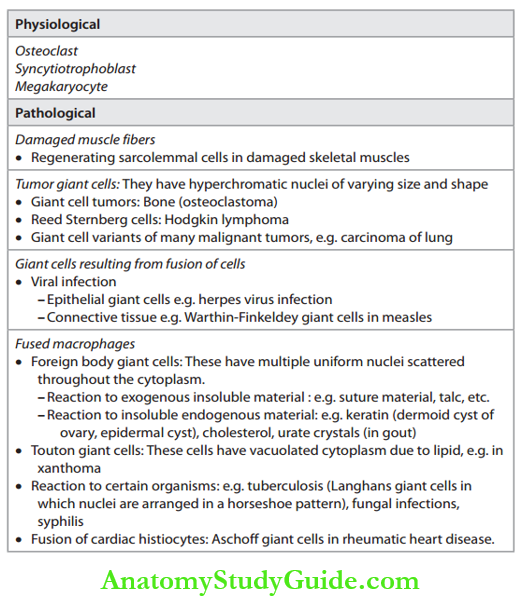
Giant Cell
Write a short note on giant cells.
Definition of Gaint cell: A cell with more than one nucleus is called a giant cell or multinucleated cell. Types of giant cells: Various types of giant cells and their associated conditions are given in Table.
Types of Gaint Cells:

Leave a Reply