Miscellaneous Viruses
Rodent-Borne Viral Infections
Rodent-borne viruses or roboviruses are transmitted from rodents to man by contact with body fluids or excretions without participation of arthropod vectors. Hence, they are not arboviruses.
They are mainly in two groups.
Table of Contents
Read And Learn More: Micro Biology And Immunology Notes
Hantaviruses
Hantaviruses are spherical, enveloped viruses; contain triple-segmented, negative-sense ssRNA, belong to Bunyaviridae.
- Rodents are the reservoir of infection
- Transmission to humans occurs by inhaling aerosols of rodent excreta.
- They cause two fatal human diseases
- Hemorrhagic fever with renal syndrome (interstitial nephritis) is caused by several species of hantaviruses such as Hantaan virus, Dobrava virus, Puumalavirus (nephropathia epidemic) and Seoul virus.
- Hantavirus pulmonary syndrome is caused by the species—Sin Nombre virus.
Arenaviruses
Arenaviruses are pleomorphic, 50–300 nm in size, enveloped with large, club-shaped peplomers and contain a segmented ssRNA (two segments).
- New world viruses: Junin, Machupo, Guanarito & Sabia viruses. They cause South American hemorrhagic fever.
- Old world viruses: Lassa fever viruses (in Africa, ribavirin is DOC) and Lymphocytic choriomeningitis (LCM) viruses.
Filoviruses
Include two genera: Ebola virus and Marburg virus both cause African hemorrhagic fever.
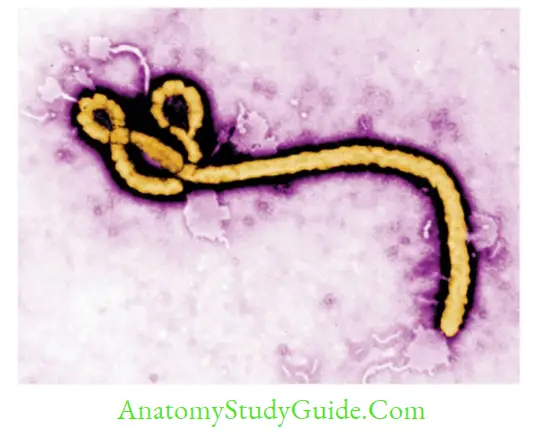
- Morphology: They are pleomorphic, and mostly appear as long filamentous, ranging from 80–1000 nm, the average size being 665 nm (Marburg) to 805 nm (Ebola).
- Highly fatal: All the viral hemorrhagic fevers, with the highest mortality rates (25–90%).
Ebola Virus
Ebola virus has become a global threat, because of its recent outbreak in 2014; which was declared by WHO, as a public health emergency of international concern.
- History: In humans, it appeared first in 1976 in Africa near the Ebola River, from which the disease takes its name.
- Species: The Ebola virus has five stable subtypes or species (Zaire, Sudan, Taï Forest, Reston, and Bundibugyo). Species are of epidemiological importance. The virus causing the 2014 West African outbreak belongs to the Zaire species.
- Geographical distribution: Since its discovery, the Ebola virus has caused several outbreaks in various African countries affecting more than 31,047 documented cases with nearly 12,889 (41%) deaths. The most recent outbreak in the world was from the Democratic Republic of the Congo (May to July 2017); reported 8 cases with 4 deaths.
- The largest outbreak occurred in 2014–16; reported 28,616 cases with 11,310 deaths (40% mortality). Three primary countries affected were—Guinea, Liberia, and Sierra Leone.
However, a few cases have also been reported from several other countries. - Reservoir: Though unknown, but are suspected to be infected animals, such as a fruit bat or primates (apes and monkeys).
- Transmission:
- Through direct close contact with the blood, secretions, organs or other body fluids of infected animals/humans, infected surfaces and materials (e.g. bedding, clothing, syringes, etc.)
- Healthcare workers and close contacts/family members are at greater risk of contracting the infection.
- Ebola can stay in semen for up to 3 months, although sexual transmission has not been reported yet.
Clinical manifestations:
- The incubation period is about 2–12 days (the average being 8–10 days)
- Common symptoms include Fever, headache, muscle pain, and sore throat, followed by abdominal pain, vomiting, diarrhea, and rash, with hemorrhages (bleeding or bruise), often leading to shock and death.
Laboratory diagnosis:
-
- Antibody detection: ELISA detects both IgM and IgG separately by using recombinant nucleoprotein (NP) and glycoprotein (GP) antigens
- Serum antigen detection by capture ELISA: The target proteins are NP, VP40, and GP.
- Molecular methods such as reverse transcriptase PCR (RT-PCR) assay and real-time
RT-PCR assay to detect the viral RNA - Electron microscopy of the specimen shows typical filamentous viruses
- Virus isolation in Vero cell line: Processing the specimen should be carried out in biosafety level-4 cabinets as there is a great risk of laboratory spread of the virus.
- Treatment: Supportive care such as rehydration is required. There is no treatment or vaccine available.
- Measures taken in India: There is no confirmed case documented yet in India.
- However, because of the risk of contracting infection from travelers, strict vigilance is going on in the airports of India.
- Any person presenting with an acute onset of fever who has been in Guinea, Liberia,
Sierra Leone or Mali in the past 21 days are kept in quarantine in the airports until tested negative for Ebola virus infection.
- Any person presenting with an acute onset of fever who has been in Guinea, Liberia,
Marburg Virus
Marburg virus disease was first reported in Germany and Yugoslavia (1967) among laboratory workers exposed to tissues of African green monkeys imported from Africa. Since then, over 450 cases have been reported in various African countries such as Kenya, South Africa, the Democratic Republic of Congo, Uganda, and Angola.
The most recent outbreak was in Angola (2005), affecting 252 people with 227 deaths (mortality rate of 90%).
Coronaviruses
Coronaviruses are enveloped; carrying petal or club-shaped peplomer spikes giving the appearance of a solar corona. Most of them infect animals and birds. Human infection is extremely rare.
- Most human coronaviruses are widespread affecting people of most of the world and produce mild upper respiratory tract infections and occasional diarrhea.
- Two exceptions are SARS-CoV and MERS-CoV which are geographically restricted, transmitted from man to man, and have produced outbreaks of severe respiratory disease with higher mortality.
- Transmission: Human coronaviruses are spread by coughing and sneezing, and close personal contact, such as touching mouth, nose, or eyes or shaking hands. SARS-CoV can also spread via droplets and rarely spread through the air (airborne spread).
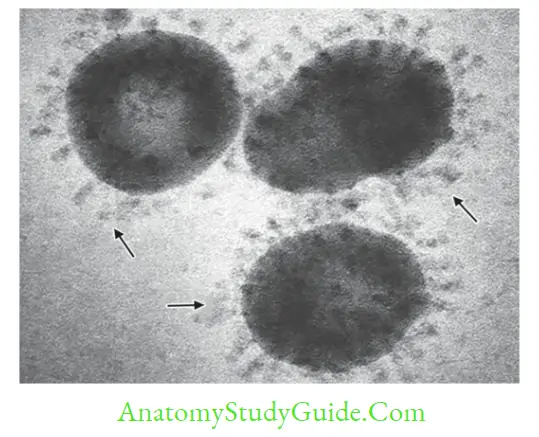
SARS-CoV (Severe Acute Respiratory Syndrome Coronavirus)
- History: SARS was first recognized in China in 2003 by WHO physician Dr. Carlo Urbani. He diagnosed it in a businessman who had traveled from China to Hong Kong, to Hanoi, Vietnam. The businessman and the doctor who was first diagnosed with SARS both died from the illness.
- Epidemiology: During the 2003 outbreak, the SARS virus, spread from Asia to various regions of the world causing nearly 8098 cases in 29 countries, with over 774 deaths in 2003.
However, India remained free from the infection. Since 2004, no case has been reported from anywhere in the world. - Source: SARS-CoV infection in humans is believed to be contracted from animals, including monkeys, Himalayan palm civets, raccoon dogs, cats, dogs, and rodents.
- Clinical manifestation includes severe lower respiratory tract infection, characterized by muscle pain, headache, sore throat, and fever, followed in 2–10 days by the onset of respiratory symptoms mainly cough, dyspnea, and pneumonia.
MERS-CoV (Middle East Respiratory Syndrome Coronavirus)
- MERS-CoV has recently caused a severe form of lower respiratory illness with a mortality of 30%.
Epidemiology: It was first reported in Saudi Arabia in 2012.
Since then, several hundreds of cases have been reported from various countries located in and around the Arabian Peninsula such as Saudi Arabia, UAE, Qatar, Oman, Jordan, Kuwait, Yemen, Lebanon, and Iran. - It is not reported from India yet.
Source though unknown, it is believed to have been acquired from camels and bats.
People at increased risk for MERS-CoV infection include: - Recent history of travel from the Arabian Peninsula within 14 days
- Close contacts of a confirmed case of MERS
- Healthcare personnel not using recommended infection control precautions
- People with exposure to infected camels.
Clinical manifestation:
- The incubation period is about 2–14 days.
- Severe acute respiratory symptoms like fever, cough, and shortness of breath may appear.
- Some people develop gastrointestinal symptoms including diarrhea and nausea/vomiting.
- Complications occur such as pneumonia and kidney failure, especially in people with underlying comorbid conditions.
Slow Viruses And Prions
Slow virus diseases including prion diseases, are a group of neurodegenerative conditions affecting both humans and animals, characterized by:
- The long incubation period, ranging from months to years
- A predilection for CNS: Slow viruses usually affect the CNS
- Cause vacuolation of neurons (spongiform changes), with deposition of amyloid-like plaques and gliosis
- Symptoms include loss of muscle control shivering, tremors, and dementia
- Invariably fatal
- Strong genetic predisposition
- Slow viruses and prions lack antigenicity; hence there are:
- Lack of immune response and interferon production against the viral proteins
- Lack of associated inflammation
- Does not produce cytopathologic effects in vitro.
Slow virus diseases are either caused by: (1) Conventional viruses or (2) Unconventional viruses termed as ‘prions’.
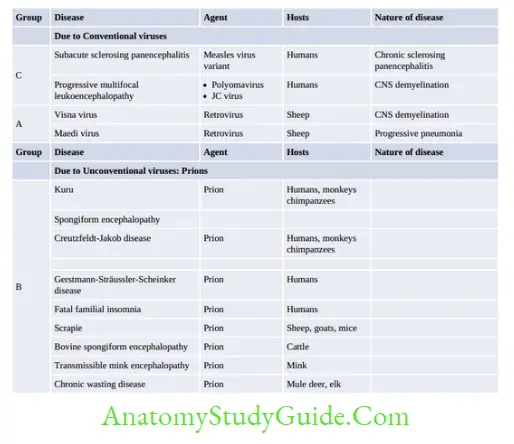
Prion Diseases
Prions are infectious protein particles that lack any nucleic acid. They are filterable like viruses but are resistant to a wide range of chemical and physical agents of sterilization. There are several
prion diseases of humans and animals.
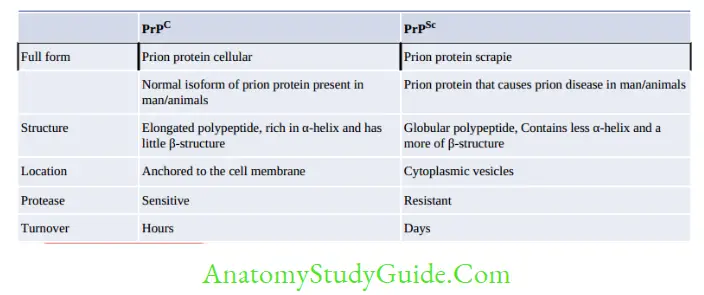
Prion Proteins have Two Isoforms
- PrPSc is the prion protein that causes disease. It is so named because it was first identified in scrapie.
- PrPC is the normal cellular isoform of the prion protein present on the cell membrane of mammals. It is encoded in chromosome 20. It is the precursor of PrPSc, they differ from each other in many respects.
Mechanism of Prion Diseases
A theory proposed by Stanley B. Prusiner (Nobel prize winner, 1997) clearly explained the detailed mechanism.
- Once infected, the prion proteins (PrPSc) are carried to the neurons. They bind to the normal PrPC on the cell surface.
- This causes the release of PrPC from the cell surface followed by their conversion into the disease-causing isoform (PrPSc). This is a post-translational modification by which the elongated polypeptide PrPC becomes globular polypeptide PrPSc.
- The cell synthesizes new PrPC and the cycle is repeated; as a result, a large amount of PrPSc is formed.
- PrPSc are aggregated as amyloid-like plaques in the brain. As these plaques consist of host proteins, there is a lack of an immune response or inflammation.
- PrPSc are internalized by neurons and get accumulated inside the cytoplasmic vacuoles giving the cell a spongiform appearance.
Laboratory Diagnosis
- Measurement of PrPSc by conformation-dependent immunoassay (definitive diagnostic tool).
- Brain MRI: > 90% of patients show increased intensity in the basal ganglia and cortical ribboning.
- Neuropathological diagnosis in brain biopsies: The pathologic hallmarks of prion diseases seen under light microscopy, are spongiform degeneration and astrocytic gliosis with a lack of inflammatory response.
- Sequencing the PRNP gene to identify the mutation: This is important in familial forms of Prion diseases.
- Stress protein 14-3-3 is elevated in the CSF.
- Abnormal EEG: In the late stage of the disease, high-voltage, triphasic sharp discharges are observed.
Treatment
There is no known effective therapy for preventing or treating prion diseases. Several trials using drugs such as quinacrine and anti-PrP antibodies have been shown to eliminate PrPSc from cultured cells, but they failed to do so in vivo.
Decontamination
Prions are extremely resistant to most of the common sterilization procedures. Recommended methods for sterilization of material contaminated with prion proteins are:
- Autoclaving at 134 °C for 1–1.5 hour
- Treatment with 1 N NaOH for 1 hour
- 0.5% sodium hypochlorite for 2 hours.
Rotavirus
Rotaviruses belong to the family Reoviridae; the only RNA virus family to have dsRNA.
- Surrounded by a triple-layered capsid
- Possess segmented dsRNA (11 segments)
- Proteins: There are 6-structural viral proteins (VPs) and 6-nonstructural proteins (NSPs).
Classification of Rotaviruses
- Traditional Classification: Based on group-specific VP6 antigen, there are seven groups (A-G) of rotaviruses. Most human diarrhea is caused by group A and, to a much lesser extent, by groups B and C.
- Binary system of typing: VP7 (a glycoprotein or G-type antigen) and VP4 (a protease-sensitive protein or P-type antigen) are used to type rotaviruses.
- Serotyping (by neutralization test) and genotyping (by sequencing) methods are available.
- Currently, 19G and 28[P] types are known. The most common type seen in the world as well as in India is the G1P [8] type, which accounts for nearly 70% of total isolates.
Clinical Manifestation
- Rotaviruses are the most common cause of diarrheal illness in children.
- IP is about 1–3 days.
- It has an abrupt onset, characterized by vomiting followed by watery diarrhea, fever, and abdominal pain.
Laboratory Diagnosis
- Direct detection of virus: Rotaviruses can be demonstrated in stool by:
- Immunoelectron microscopy (IEM): Rotaviruses have sharp-edged triple-shelled capsids; that look like spokes grouped around the hub of a wheel.
- Isolation of rotavirus is difficult. Rolling of tissue cultures may be attempted to enhance replication.
- Detection of viral antigen in stool- by ELISA and latex agglutination-based methods.
- RT-PCR is the most sensitive detection method for the detection of rotavirus from the stool.
- Serologic tests (ELISA) can be used to detect the rise of antibody titers. This may be useful for seroprevalence purposes.
Treatment and Control
Treatment is mainly supportive, to correct the loss of water and electrolytes such as oral or parenteral fluid.
Vaccine: Two brands of rotavirus vaccine are available:
- Rotavac: It contains live attenuated Rotavirus 116E (G9P[10] strain). It provides cross-protection against many types including G1P[8] type.
- It is manufactured by Bharat Biotech, India
- It is introduced under the national immunization schedule of India in selected states
- Three doses (0.5 mL, i.e. 5 drops/dose): administered at 6, 10, and 14 weeks along with DPT and OPV
- Overall efficacy in the first 2 years of life is about 55%
- Side effects (≥5%): crying, irritability, fever, and diarrhea. No vaccine-induced intussusception has been reported.
- Rotarix: Rotarix contains live attenuated G1P[8] strain; also provides cross-protection against G3, G4, and G9.
It has to be reconstituted before use. Given in two doses: 1st at 6 weeks and 2nd dose is given 4 weeks later.

Leave a Reply