Obturation Of Root Canal System Notes
The success of endodontic treatment is based on proper diagnosis, treatment planning, and biomechanical preparation followed by obturation. Obturate means to fill the shaped and disinfected canal with a temporary or permanent filling material.
Table of Contents
It can be achieved by using cement, pastes, plastics, or solids. Gutta-percha, in its various forms, has remained the material of choice for obturation.
Why To Obturate
Microorganisms and their byproducts are the major cause of pulpal and periapical diseases. However, it is difficult to consistently and totally disinfect root canal systems. Therefore, the goal of three-dimensional (3-D) obturation is to provide an impermeable fluid-tight seal within the entire root canal system, to prevent oral and apical microleakage.
Read And Learn More: Endodontics Notes
Objectives of obturation are:
- Elimination of coronal leakage of microorganisms or potential nutrients to support their growth in dead space of root canal system
- To confirm any residual microorganisms that have survived the chemomechanical cleaning and shaping, to prevent their proliferation and pathogenicity
- To prevent the percolation of periapical fluids into the root canal system and feeding microorganisms
History:
- 1757—Carious teeth were extracted, filed with gold/lead, and replanted again.
- 1847—Hill’s stopping was developed.
- 1867—CA Bowman claimed to be the first to use gutta-percha for root canal filling.
- 1883—Perry claimed that he had been using a pointed gold wire wrapped with some gutta-percha (roots of present-day core carrier technique).
- 1887— SS White Company began to manufacture GP points.
Timing Of Obturation
Patient Symptoms:
Sensitivity on percussion—indicates inflammation of periodontal ligament space, hence canal should not be obturated before the inflammation has subsided.
Pulp and Periradicular Status:
Vital Pulp Tissue:
In the case of vital pulp, obturation can be done in a single visit after complete cleaning and shaping
Necrotic Pulp Tissue:
- Single-visit endodontics can be done if the tooth is asymptomatic
- If a patient presents with sensitivity on percussion, it indicates inflammation of periodontal ligament space, hence canal should be obturated after the inflammation has subsided.
Purulent Exudates:
If obturation is done in the tooth with purulent exudate, pressure, and subsequent tissue destruction may occur rapidly. In such cases, calcium hydroxide should be placed as an intracanal medicament.
Negative Culture:
Dependence on negative culture has decreased now because studies have shown that false negative results can give an inaccurate assessments of microbial flra; also the positive results do not indicate the potential pathogenicity of bacteria.
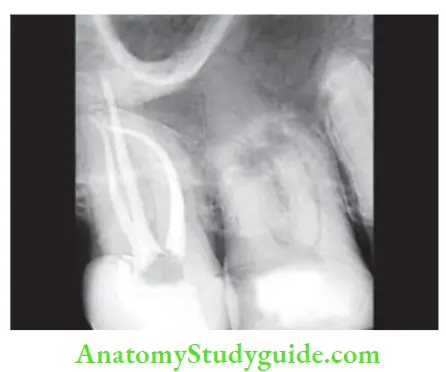
Extent Of Root Canal Filling
- The anatomic limit of pulp space is cementodentinal junction (CDJ) apically and pulp chamber coronally. Kuttler (1995) described CDJ as a minor apical diameter which ends 0.5 mm short of an apical foramen in young patients and 0.67 mm short in older patients.
- According to Cohen, the apical point of termination should be 1 mm from the radiographic apex. Radiographically, the root canal filling should have the appearance of a dense, 3-D filing up to cementodentinal junction.
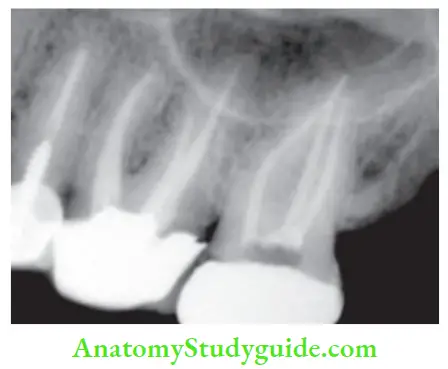
So ideal obturation should:
- Fill the entire root canal three-dimensionally as close to CDJ as possible
- Reflect a continuously tapered funnel same as external root morphology
- Radiographically appear as 3-D filing that extends close to CDJ
Overextended obturation is the vertical dimension of root filing beyond the apex.
Overfilling is total obturation of the root canal system with excess material extruding beyond the apical foramen.
Underfiling is the filing of root canal system >2 mm short of radiographic apex.
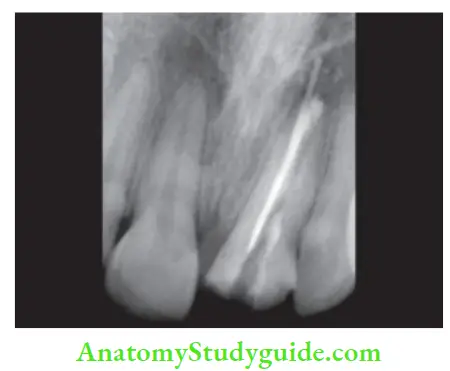
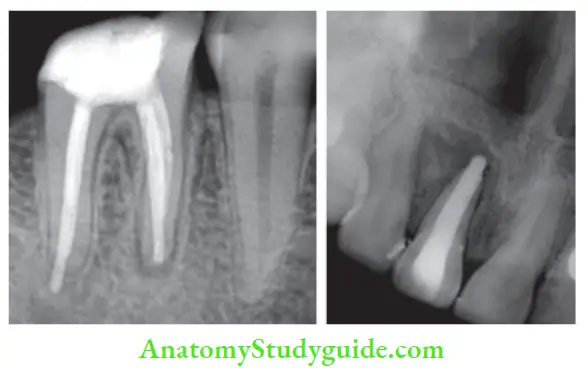
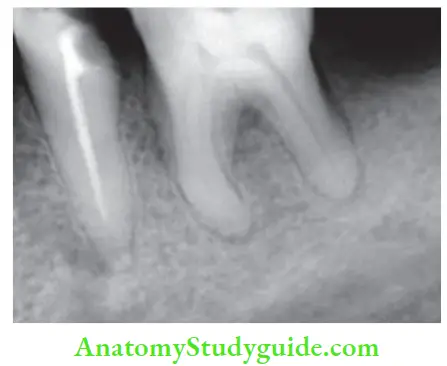
Evaluation of obturation:
Radiographically, an obturated tooth should show
- Three-dimensionally failed root canal
- Dense radiopaque filing of the root canal system
- Filling close to apical terminus without overextending periodically
But there can be difficulty in radiographic interpretation due to the radiopacity of the sealer, overlying bony anatomy, and 2-D view.
Materials Used For Obturation
An ideal root canal filling should be capable of completely preventing communication between the oral cavity and periapical tissue. Root canal sealers should be biocompatible or well tolerated by the tissues in their set state and are used in conjunction with the core filing material to establish an adequate seal.
Grossman (1982) grouped acceptable filing materials into plastics, solids, cement, and pastes. He gave the following 10 requirements for an ideal root canal filling material:
- Easily introduced into a root canal
- Seal the canal laterally as well as apically
- Not shrink after being inserted
- Impervious to moisture
- Bacteriostatic or at least do not encourage bacterial growth
- Radiopaque
- Nonstaining the tooth structure
- Nonirritating
- Sterile/easily sterilized immediately before obturation
- Easily removed from the root canal if necessary
Materials used for root canal obturation are:
- Silver cones
- Gutta-percha
- Custom cones
- Resilon
- Root canal sealers
Silver Cones:
- Jasper (1941) introduced silver cones with the same success rate as gutta-percha and easier to use
- The rigidity provided by the silver cones made them easy to place and permitted length control
- Due to the stiffness of silver cones, these were mainly used for teeth with fie, tortuous, and curved canals like canals of maxillary first premolars, mesial canals of mandibular molars
- Since silver cones lack plasticity, these are not used in oval canals like single canal premolars and teeth with oval canals in young persons
- But nowadays their use has declined, due to corrosion caused by them. The presence of traces of copper, nickel, etc. in silver points adds up the corrosion
Gutta-Percha:
Gutta-percha is derived from two words:
- “GETAH”—meaning gum
- “PERTJA”—the name of the tree in the Malay language
Gutta-percha was initially used as a restorative material and later developed into an indispensable endodontic filling material.
Being biologically inert, resilient, and electric insulator, it was used for various purposes such as coating the transatlantic telegraph cable, cores of golf balls, handles of knives, splints for holding fractured joints, to control hemorrhage in extracted sockets, in various skin diseases such as psoriasis and eczema.
Historical background:
- 1843—Sir Jose d’Almeida first introduced gutta-percha to the Royal Society of England.
- In Dentistry—Edwin Truman introduced gutta-percha as temporary filing material.
- 1847—Hill introduced Hill’s stopping (a mixture of bleached gutta-percha and carbonate of lime and quartz).
- 1867—Bowman first used gutta-percha as root canal filling material.
- 1883—Perry packed gold wire wrapped with gutta-percha in root canals.
- 1887—SS White Company started the commercial manufacture of gutta-percha points.
- 1893—Rollins used gutta-percha with pure oxide of mercury in root canals.
- 1914—Callahan softened the gutta-percha by using rosins and then used it for obturation of the root canals.
- 1959—Ingle and Levine proposed standardization of root canal instruments and filing materials.
- 1976—A group evolved into present-day ISO for approval of the specification of root canal instruments and filing materials. ADA specification for gutta-percha points is no. 78
Sources:
Gutta-percha is a rigid natural latex produced from the sap of trees of the genus Palaquium. These trees are found in Southeast Asia, especially in Malaysia and Indonesia. In India, these are found in Assam and Western Ghats.
Chemistry:
It is an isomer of natural rubber and the natural chemical form of gutta-percha is trans-1,4-polyisoprene. Th cis-form belongs to the latex elastomer.

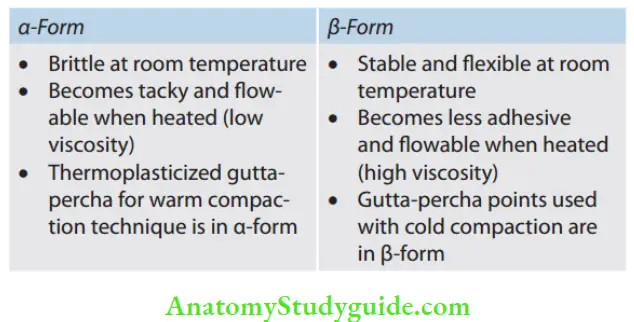
Phases of gutta-percha:
Chemically pure gutta-percha exists in two different crystalline forms, that is, α and β, which differ in molecular repeat distance and single bond form. Natural gutta-percha coming directly from the tree is in α-form while the most commercially available product is in β-form. These phases are interconvertible.
Properties of Gutta-percha:
- Biocompatibility: It is inert, highly biocompatible
- Ductility: Depending on phase existence, it is a ductile material
- Melting point: It is 60°C
- Dimensional stability: Expansion and shrinkage occurs due to heating and cooling, otherwise it is a dimensionally stable material
- Ease of handling: It can be used both in α- and β-form for obturation depending upon handling features
Clinical Considerations:
- On heating, gutta-percha expands which accounts for its increased volume which can be compacted into the root canal
- Gutta-percha shrinks as it returns to normal temperature. So, vertical pressure should be applied in all warm guttapercha techniques to compensate for volume change when cooling occurs (Schilder et al.)
- The aging of gutta-percha causes brittleness because of the oxidation process. Storage under artificial light also speeds up their deterioration. Brittle guttapercha can be rejuvenated by a technique described by Sorien and Oliet. In this, gutta-percha is immersed in hot water (55°C) for 1 or 2 s and then immediately immersed in cold water for a few seconds
- Gutta-percha cannot be heat sterilized. For disinfection of gutta-percha points, they should be immersed in 5.25% NaOCl for 1 min
- After this, gutta-percha should be rinsed in hydrogen peroxide or ethyl alcohol to remove crystallized NaOCl before obturation, as these crystallized particles impair the obturation
- Gutta-percha should always be used with sealer and cement to seal root canal space as gutta-percha lacks adhering qualities
- Gutta-percha is soluble in certain solvents like chloroform, eucalyptus oil, etc. This property can be used to plasticize gutta-percha by treating it with solvent for better filing in the canal. But it has shown that gutta-percha shrinks (1–2%) when solidified
- Gutta-percha also shows some tissue irritation which is due to the high content of zinc oxide
Current Available Forms of Gutta-percha:
- Gutta-percha points: Standard cones are of the same size and shape as that of ISO endodontic instruments
- Auxiliary points: Non-standardized cones; perceive the form of root canal
- Greater taper gutta-percha points: Available in 4%, 6%, 8%, and 10% taper
- Gutta-percha pellets/bars: They are used in thermoplasticized gutta-percha obturation, for example, obtura system
- Precoated core carrier gutta-percha: In these stainless steel, titanium or plastic carriers are precoated with α-phase gutta-percha for use in the canal, for example, thermal
- Syringe systems: They use low viscosity gutta-percha, for example, α-seal
- Gutta flow: In this gutta-percha, the powder is incorporated into the resin-based sealer
- Gutta-percha sealers like chloropercha and eucopercha: In these, gutta-percha is dissolved in chloroform/ eucalyptol to be used in the canal
- Medicated gutta-percha: Calcium hydroxide, iodoform, or chlorhexidine diacetate containing gutta-percha points
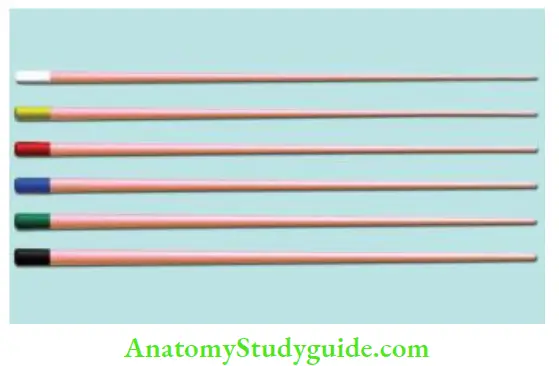
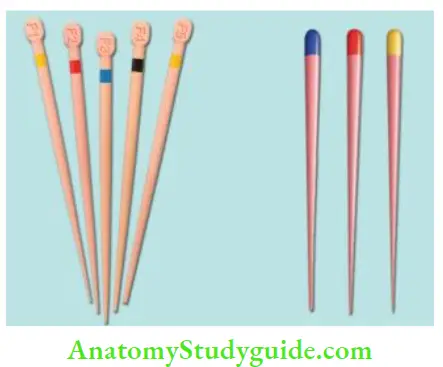
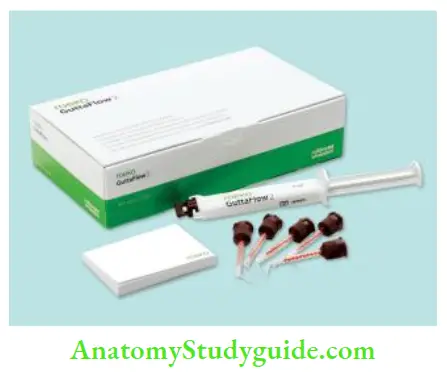
Advantages of gutta-percha:
- Compatibility: Adaptation to canal walls
- Inertness: Makes it nonreactive material
- Dimensionally stable
- Tissue tolerance
- Radiopacity: Easily recognizable on the radiograph
- Plasticity: Becomes plastic when heated
- Dissolve in some solvents like chloroform, eucalyptus oil, etc. This property makes it more versatile as canal filling material
Disadvantages of gutta-percha:
- Lack of rigidity: Bending of gutta-percha is seen when lateral pressure is applied. So, difficult to use in smaller canals
- Easily displaced by pressure
- Lacks adhesive quality
Medicated Gutta-percha:
Calcium hydroxide containing gutta-percha: These are available in ISO size of 15–140 and are made by combining 58% of calcium hydroxide in a matrix of 42% gutta-percha. The action of calcium hydroxide is activated by moisture in the canal
Advantages of calcium hydroxide points:
- Ease of insertion and removal
- Minimal or no residue left
- Firm for easy insertion
calcium hydroxide points Disadvantages:
- Short-lived action
- Radiolucent
- Lack of sustained release
- Calcium hydroxide plus points
- Along with calcium hydroxide and gutta-percha, they contain tenside which reduces the surface tension
- Due to the presence of water-soluble components, such as tenside and sodium chloride, they are three times more reactive than calcium hydroxide points
- They have superior pH and increase the wettability of canal surface with increased antibacterial property
- They have sustained alkaline pH for one week
- Iodoform containing gutta-percha:
- Iodoform containing gutta-percha remains inert till it comes in to contact with the tissue fluids
- On coming in to contact with tissue fluids, free iodine is released, which is antibacterial in nature
- Chlorhexidine diacetate containing gutta-percha:
- In this, the gutta-percha matrix is embedded in 5% chlorhexidine diacetate
- Ths material is used as an intracanal medicament
Resilon:
- A resin-based obturation system was introduced as an alternative to gutta-percha. It consists of a resin core material (Resilon) composed of polyester, difunctional methacrylate, bioactive glass, and radiopaque filers, and a resin sealer. The core material is available in conventional and standardized cones and pellets
- Resilon core bonds to resin sealer, which attaches to the etched root surface forming a “monoblock.” This results in a gutta-percha–sealer interface and a tooth–sealer interface. This bonding provides a better coronal seal and may strengthen the root Resilon is discussed in detail on page no. 290.
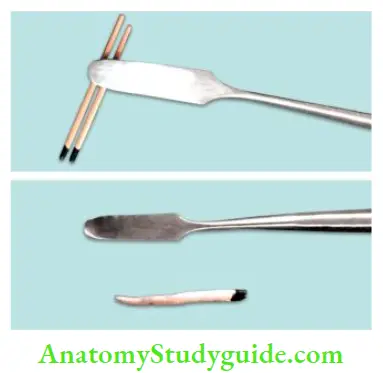
Custom Cones:
- When the apical foramen is open or the canal is large, a custom cone is made which allows adaptation of the cone to canal walls and improves the seal
- The technique involves the customization of gutta-percha cones according to the canal shape by:
- Softening it in chloroform, eucalyptol, or halothane for 1–2 seconds
- Heating several large gutta-percha cones and rolling the mass between two glass slabs until an appropriate size is obtained
- The softened cone is then placed into the canal and gently packed to the length. This process is repeated until an adequate impression of the canal is obtained at the prepared length
Root Canal Sealers:
The purpose of sealing root canals is to prevent periapical exudates from diffusing into the unfiled part of the canal, to avoid re-entry and colonization of bacteria, and to check residual bacteria from reaching the periapical tissues.
Therefore to accomplish a fluid tight seal, a root canal sealer is needed. Though sealer is used only as adjunct material in obturation, it affects the prognosis of endodontic treatment. An adequate combination of sealing ability and biocompatibility of root canal sealer is important for a favorable prognosis of root canal treatment.
Requirements of an Ideal Root Canal Sealer:
According to Grossman, a root canal sealer should be
- Tacky when mixed so as to provide good adhesion between it and the canal wall
- Able to create a hermetic seal
- Radiopaque so as to be visible on a radiograph. According to ANSI/ADA specification number 57, all endodontic sealers should be at least 2 mm Al more radiopaque than dentin or bone
- Of very fine powder particles for the optimal mix
- Not shrink upon setting
- Nonstaining to tooth structure
- Bacteriostatic or at least do not encourage bacterial growth
- Set slowly because long working time allows placement and adjustment of root filing if necessary
- Insoluble in tissue fluids
- Nonirritating to periarticular tissue
- Soluble in a common solvent so that it can be removed from the root canal if required
The following were added to Grossman’s basic requirements:
- It should not provoke an immune response in periarticular tissue
- It should be neither mutagenic nor carcinogenic
Functions of Root Canal Sealers:
- Antimicrobial agent: Sealers show an antibacterial effect which is excreted immediately after their placement
- Fill in the discrepancies between the obturating material and dentin walls
- Binding agent: Acts as a binding agent between obturating material and dentin walls
- As lubricant: When used with semisolid materials, sealer acts as a lubricant
- Radiopacity: Due to radiopacity, sealer can be seen on radiograph and thus can show the presence of auxiliary canals, resorptive areas, root fractures, and shape of the apical foramen
- Certain techniques dictate the use of a particular sealer: For example, chloropercha technique uses material as a sealer as well as a solvent for the master cone. It allows the shape of normal gutta-percha cone to be altered according to the shape of the prepared canal
Classification:
Classification of sealers According to their Composition
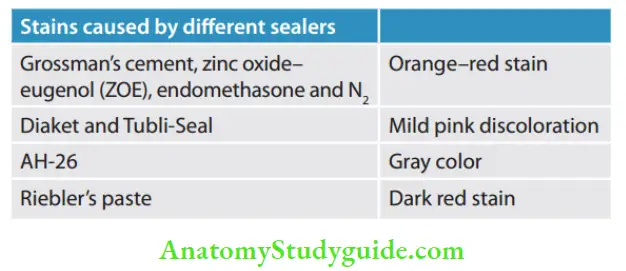
- Eugenol
- Silver containing cement:
- Kerr sealer (Rickert, 1931)
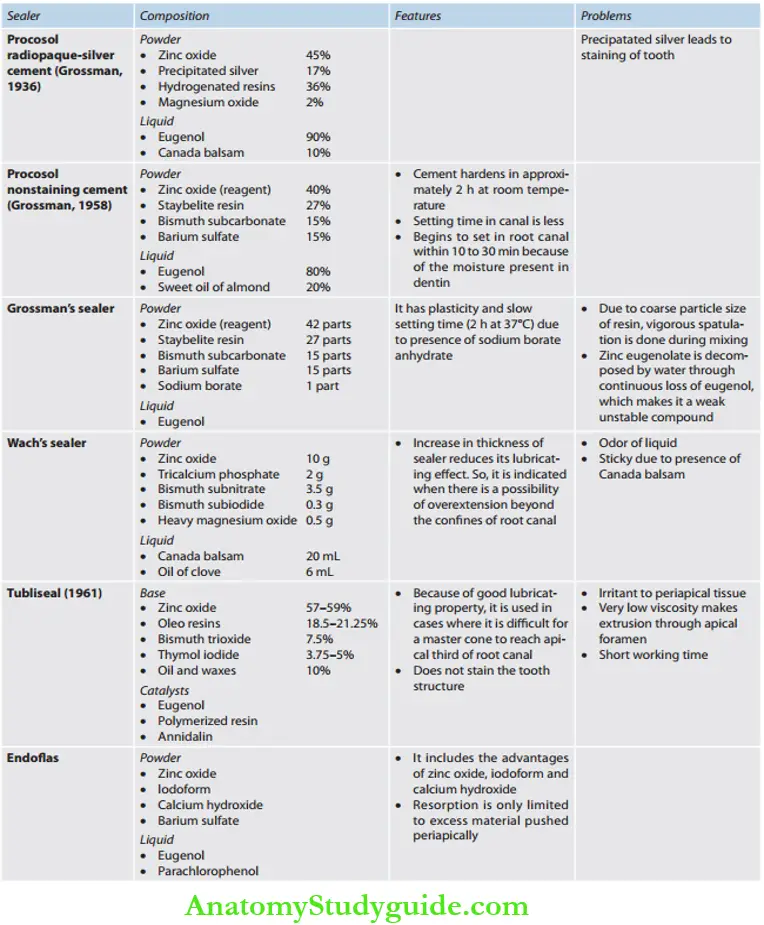
- Procosol radiopaque silver cement (Grossman, 1936)
- Silver-free cements:
- Procosol nonstaining cement (Grossman, 1958)
- Grossman’s sealer (Grossman, 1974)
- Tubliseal (Kerr, 1961)
- Wach’s paste (Wach)
- Noneugenol
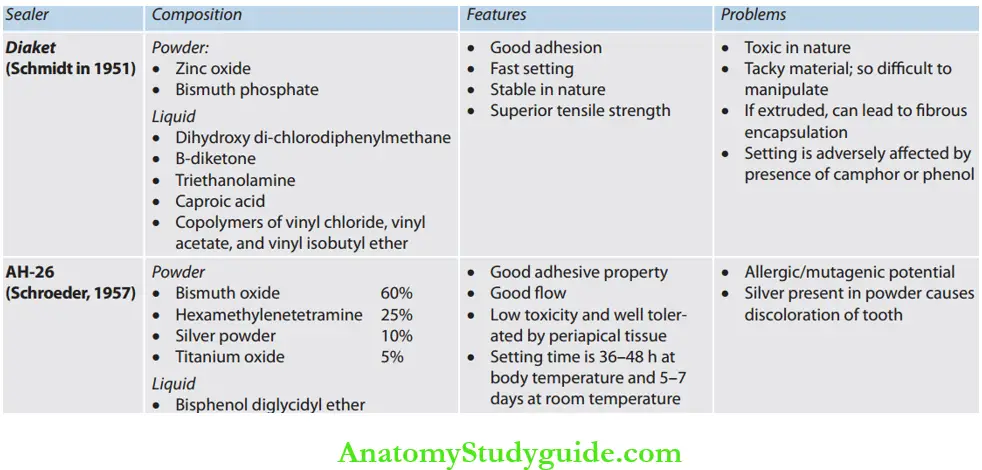
- Diaket
- AH-26
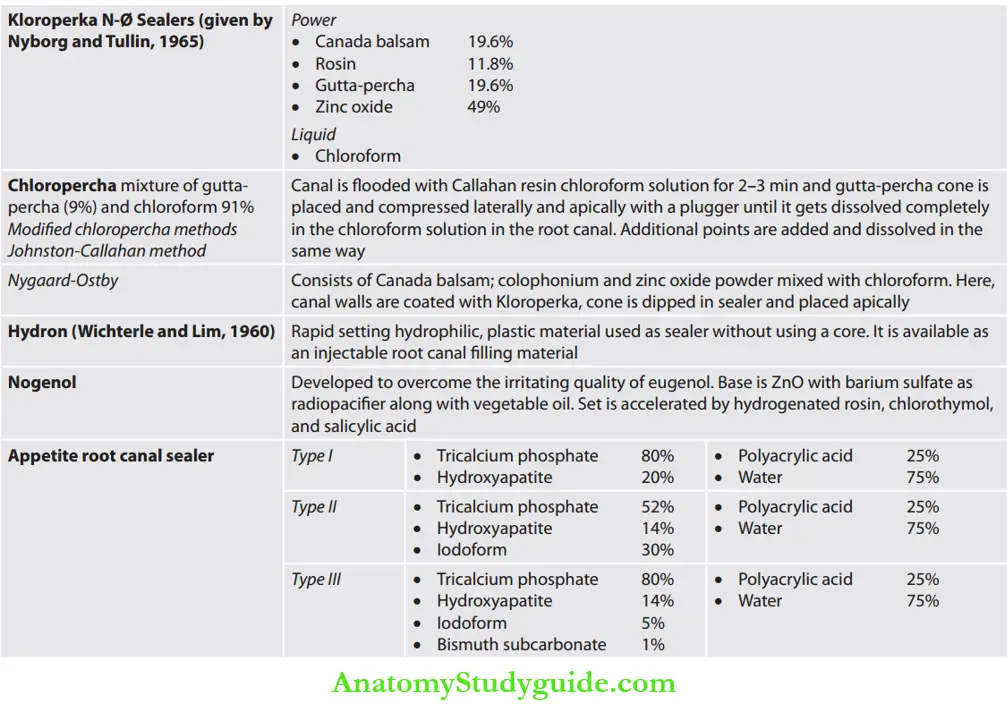
- Chloropercha and eucapercha
- Nogenol
- Hydron
- Endofi
- Glass ionomer
- Polycarboxylate
- Calcium phosphate cement
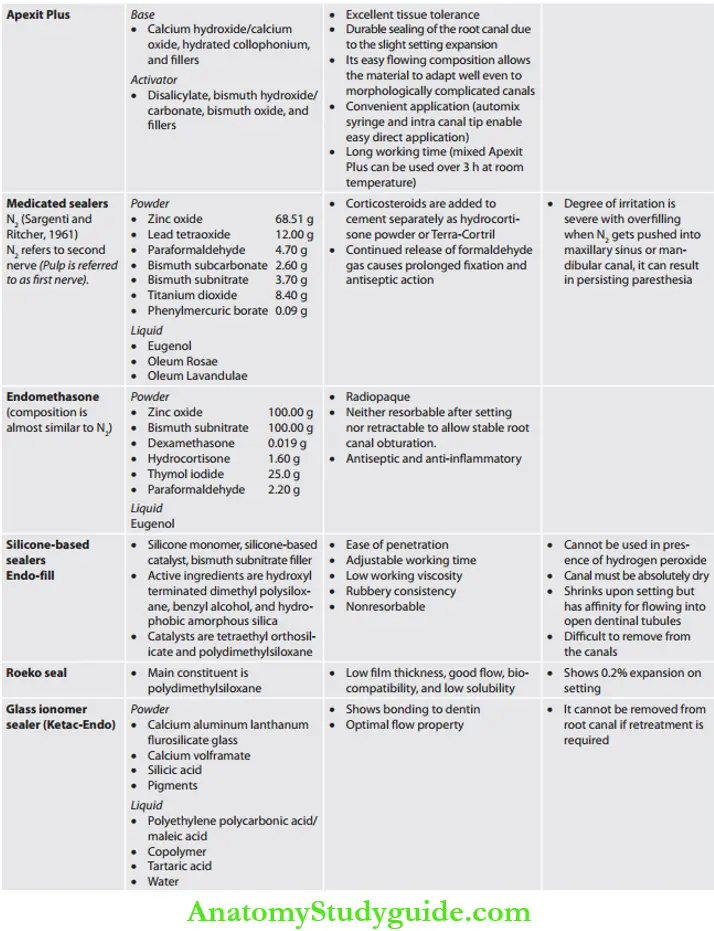
- Medicated: These sealers have therapeutic properties and are normally used without core materials
- Diaket-A
- N2
- Endomethasone
- SPAD
- Iodoform paste
- Riebler’s paste
- Mynol cement
- Ca(OH)2 paste
Classifiation According to Grossman:
- Zinc oxide resin cement
- Calcium hydroxide cement
- Paraformaldehyde cement
- Pastes
According to Cohen:
ADA specification no. 57 classifies endodontic filling materials as follows:
- Type 1: Material intended to be used with core material Subtypes:
- Class 1: Includes materials in the form of powder and liquid that sets through a nonpolymerizing process
- Class 2: Includes material in the form of two pastes that sets through a nonpolymerizing process
- Class 3: Includes polymers and resin systems that set through polymerization
- Type 2: Material intended to be used with or without core material or sealer
- Class 1: Powder and liquid nonpolymerizing
- Class 2: Paste and paste nonpolymerizing
- Class 3: Metal amalgams
- Class 4: Polymer and resin systems—polymerization
According to Ingle:
- Cement
- Pastes
- Plastics
According to Harty FJ:
- ZOE-based
- Resin-based: Consists of an epoxy resin base which sets upon mixing with an activator. For example, AH-26, Diaket, hydron
- Gutta-percha-based cement consists of solutions of gutta-percha in organic solvents, for example, , Superga
- Dentin adhesive materials, like cyanoacrylate cement, glass ionomer cement, polycarboxylate cement, calcium phosphate, composite materials
- Materials to which medicaments have been added; these are divided into two groups:
-
- In which strong disinfectants are added to decrease possible postoperative pain, like paraformaldehyde and corticosteroid preparation
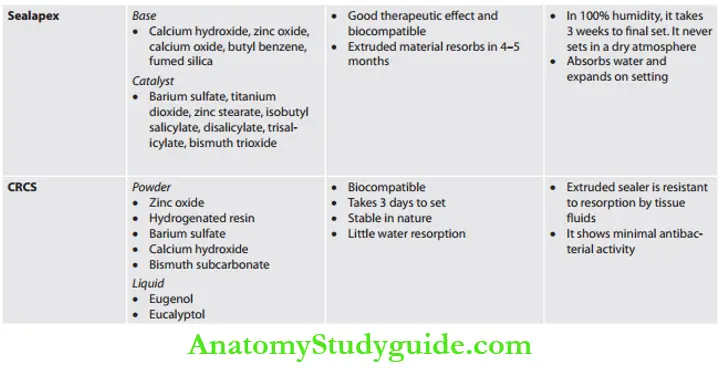
- Those in which calcium hydroxide is to induce cementogenesis and dentinogenesis at the foramen, thus creating a permanent biological seal. For example, antibiotic root canal sealer (CRCS), sealapex, and biocalex.
Zinc Oxide Eugenol (ZOE) Sealers:
Chisolm in 1873 introduced zinc oxide and oil of clove cement in dentistry.
Setting reaction:
It sets because of a combination of physical and chemical reactions, yielding a hardened mass of zinc oxide embedded in a matrix of long sheath-like crystals of zinc eugenol. The hardening of the mixture is due to the formation of zinc eugenol.
The presence of free eugenol tends to weaken the set and shows cytotoxicity. Practically, all ZOE sealer cement are cytotoxic and invoke an inflammatory response in connective tissue.

Root Canal Sealers without Eugenol:
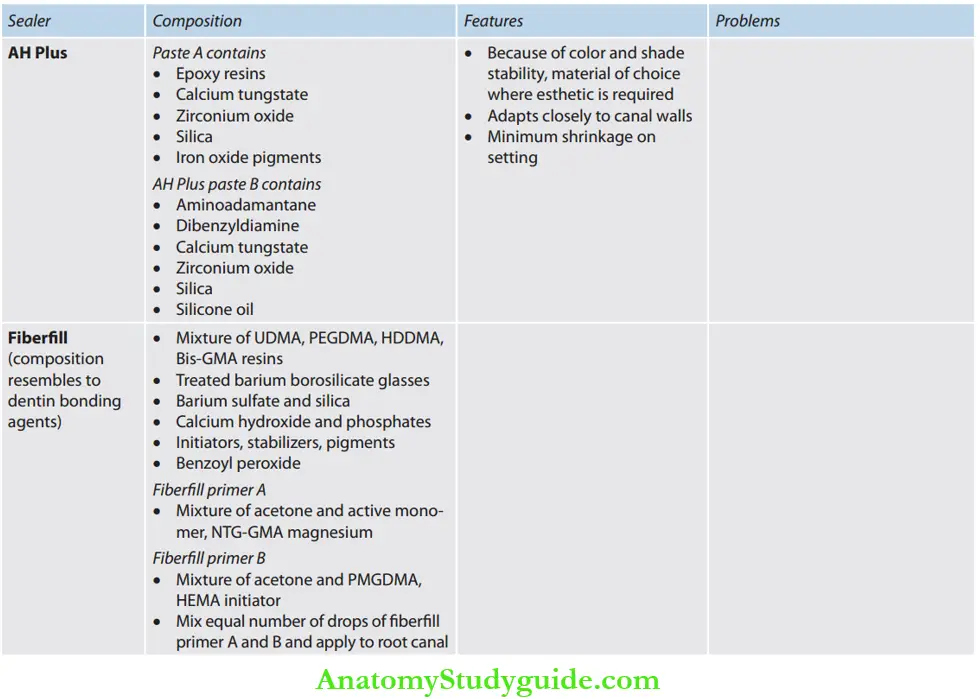
Resin-Based Sealers:
Calcium Hydroxide Sealers:
Resilon:
Resilon (Epiphany, Pentron Clinical Technologies; Wallingford, CT; RealSeal, SybronEndo; Orange, CA) is developed to overcome the problems associated with guttapercha, viz
- Shrinkage of gutta-percha on cooling
- Gutta-percha does not bind physically to the sealer, it results in gap formation between the sealer and the gutta-percha
Resilon core shrinks only 0.5% and bonds to sealer by polymerization, so no gaps are seen. It is biocompatible, noncytotoxic, and nonmutagenic. The excellent sealing ability of resin system is attributed to the “monoblock”
which is formed by the adhesion of the resin cone to the epiphany sealer, which adheres and penetrates into the dentin walls of the root canal system.
Components of Resilon System:
1. Primer:
It is a self-etch primer, which contains a sulfonic acid terminated functional monomer, HEMA, water, and a polymerization initiator
2. Resilon sealer:
It is a dual-cure, resin-based sealer. The resin matrix contains Bis-GMA, ethoxylated Bis-GMA, UDMA, and hydrophilic difunctional methacrylates. It contains filers of calcium hydroxide, barium sulfate, barium glass, bismuth oxychloride, and silica. Total fiber content is 70% by weight
3. Resilon core material:
It is a thermoplastic synthetic polymer-based (polyester) core material which contains bioactive glass, bismuth oxychloride, and barium sulfate. Filler content is 65% by weight
Method of Use:
1. Smear layer removal:
Sodium hypochlorite should not be the last irrigant to be used due to compatibility issues with resins. Use 17% EDTA or 2% chlorhexidine as a fial rinse
2. Placement of primer:
After drying the canal using paper points, primer is applied up to the apex. Use dry paper points to wick out the excess primer from the canal. Primer is very important because it creates a collagen matrix that increases the surface area for bonding. Low-viscosity primer also draws the sealer into the dentinal tubules
3. Placement of sealer:
The sealer can be placed into a canal using a lentil spiral or by coating the master cone
4. Obturation:
Obturate the canals by lateral or warm vertical compaction
5. Curing:
Resilon is cured with a halogen curing light for 40 s
6. Coronal restoration:
A coronal restoration is done to seal the access cavity
Advantages of epiphany:
- Biocompatible
- Good coronal seal; so less microleakage
- Nontoxic
- Nonmutagenic
- Forms monoblock
- Increases resistance to fracture in treated teeth
The disadvantage of epiphany:
- Does not retain its softness after heating
Monoblock Concept:
The literal meaning of monoblock is a single unit. The Monoblock concept means the creation of a solid, bonded, continuous material from one dentin wall of the canal to the other. The Monoblock phenomenon strengthens the root by approximately 20%.
Classifiation of the monoblock concept based on the number of interfaces present between the core filling material and bonding substrate:
Primary: In this, obturation is completely done with the core material, for example, use of Hydron, MTA, and BioGutta as en masse materials.
Secondary: They has two circumferential interfaces, one between the sealer and the primed dentin and the other between the sealer and core material. For example, a resin-based system.
Tertiary: In this, the conventional gutta-percha surface is coated with resin which bonds with the sealer, which further bonds to canal walls. So, there are three circumferential interfaces
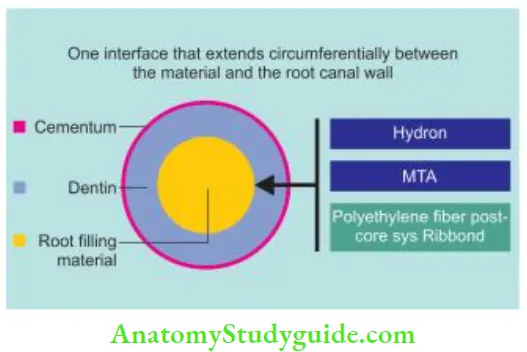
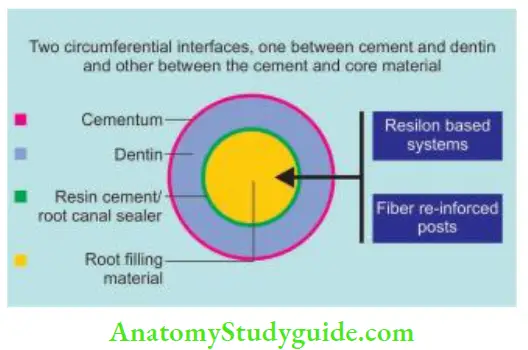
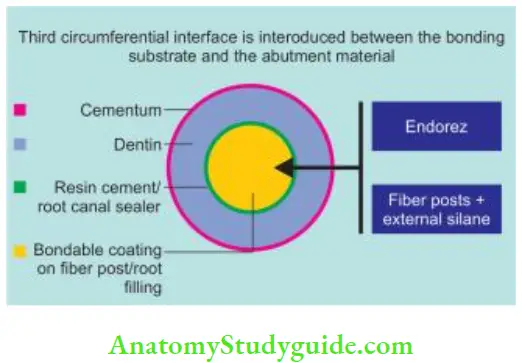
- Between the sealer and primed dentin
- Between the sealer and coating which has been applied over gutta-percha to make them bondable to the root surface
- Between the coating and the core material, For example, EndoRez and Activ GP system.
Two prerequisites for a monoblock to function as a mechanically homogenous unit:
- The material should be able to bond strongly and mutually to each other and the substrate used for monoblock
- Monoblock material should have the same modulus of elasticity as that of the substrate (dentin/restoration)
Methods Of Sealer Placement
- Coating the master cone and placing the sealer in the canal with a pumping action.
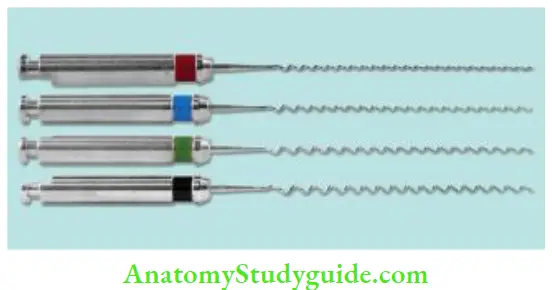
- Placing the sealer in the canal with a lentil spiral
- Placing the sealer on the master apical fie and turning the fie counterclockwise
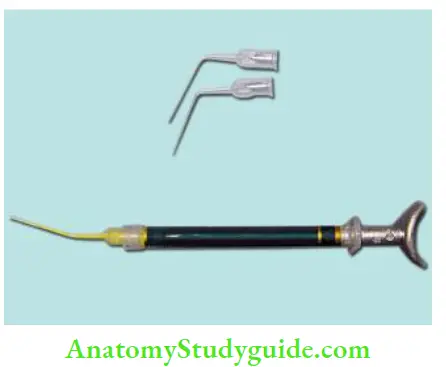
- Injecting the sealer with special syringes
- Sealer placement techniques vary with the status of the apical foramen
- If the apex is open, only apical one-third of the master cone is coated with sealer to prevent its extrusion into periapical tissues
- If the apex is closed, any of the above techniques can be used
Leave a Reply