Oncology
Cell Cycle
Question 1. Describe briefly about cell cycle and its regulation.
Answer: Cell replication proceeds through a number of phases that are biochemically initiated by external stimuli and modulated by both external and internal growth controls.
Phases of Cell Cycle

Read And Learn More: General Medicine Question And Answers
Regulators of Cell Cycle
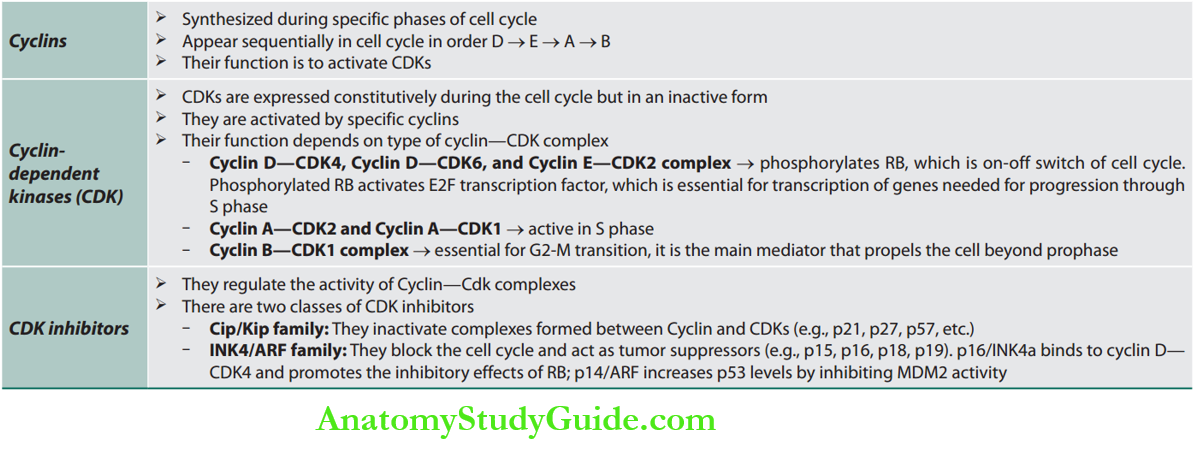
Cell Cycle Checkpoints

Question 2. Write a short essay/note on:
Answer:
- Proto-oncogene, oncogene, and tumor suppressor genes.
Regulation of Cancer Cell Growth
Normal cells may undergo malignant transformation by corrupting any one of the normal steps involved in cell proliferation.
Abnormal regulation of cell growth in cancer can occur as the result of several mechanisms.
All cancers show eight fundamental changes in cell physiology.
These are considered the hallmarks of cancer and include:
Self-sufficiency in growth signals (activation of cell growth):
- Cancers use multiple mechanisms to sustain their proliferation:
- Tumors may produce growth factors for which they have receptors in an autocrine fashion.
- Tumors may stimulate surrounding normal tissues and these normal tissues provide growth factors for the tumor.
- The tumor may become hypersensitive to growth factors through the upregulation of growth receptors or alterations of the structure of these receptors.
- Finally, they may become independent of growth factors by the presence of somatic mutations activating downstream pathways for example, BRAF mutations activating the mitogen-activated
protein kinase (MAPK) pathway, or mutations in phosphoinositide-3-kinase (PI3K) leading to activation of the PI3K/Akt/mTOR pathway, or by altering the negative feedback loops.
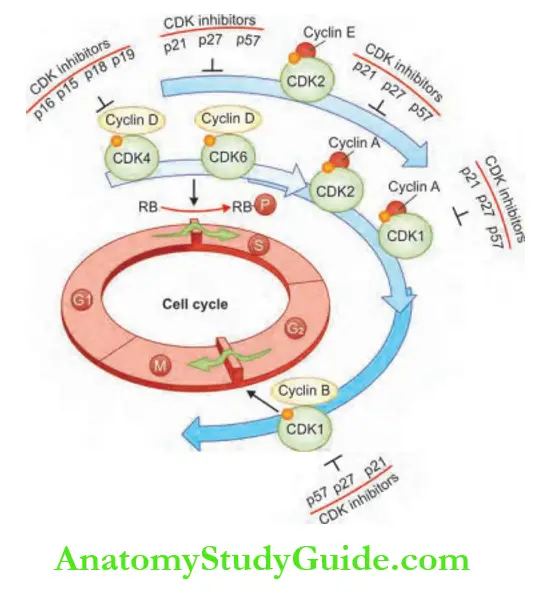
Proto-oncogenes, Oncogenes
Proto-oncogenes are normal cellular genes, which encode a number of nuclear proteins that regulate normal cell proliferation, differentiation, and survival.
Genes that promote autonomous cell growth in cancer cells are called oncogenes and are altered/mutated versions of proto-oncogenes.
Oncogenes and oncoproteins: Oncogenes can promote cell growth in the absence of normal growth-promoting/ mitogenic signals.
Oncoproteins are products of oncogenes and resemble the normal products of proto-oncogenes.
Oncoproteins production is not under normal regulatory control. Cells proliferate without the usual requirement for external signals and are freed from checkpoints and growth becomes autonomous.
Oncogenes are classified depending on the function of the gene product (oncoprotein).
Tumor Suppressor Genes
2. Insensitivity to growth-inhibitory signals (inhibition of tumor suppressor genes):
The second mechanism of carcinogenesis results from failure of growth inhibition, due to deficiency of normal tumor suppressor genes and their products.
Tumor suppressor genes apply brakes to cell proliferation and prevent uncontrolled/abnormal cell proliferation and induce repair or self-death (apoptosis).
Important tumor suppressor genes are RB, p53, BRCA1, and BRCA2 genes.
- The RB protein is responsible for the control of the cell cycle and the switch from resting state to cell division, mainly in response to the stimuli outside of the cell.
- The cells that lack the RB protein do not have this control mechanism.
- The p53 protein is also a cell cycle control protein, but it responds mainly to intracellular stressors, and it can stop the cell cycle until the abnormal processes have been corrected.
- the p53 protein arrests cells in the quiescent G1 and G2 phases of the cell cycle, preventing cells from entering the DNA synthetic (S) phase of the cell cycle.
- It can detect DNA abnormalities, such as nucleotide mismatches and DNA strand breaks, including those caused by radiation and chemotherapy.
- The function of p53 is thought to be critical in preserving the integrity of the cellular genome.
- The NF2 gene and its product Merlin. Merlin is responsible
for maintaining contact inhibition through E-cadherins. - Normal cells stop proliferating when a desired density of cells is achieved.
- This process is dysregulated in cancer (loss of contact inhibition).
- The LKB1 epithelial polarity protein is responsible for maintaining tissue integrity and contributes to the contact inhibition phenomenon.
- The functional LKB1 can even overcome signals originating from strong oncogenes such as Myc.
- Tumor growth factor-β (TGF-β) functions as a suppressor of tumor growth, but in advanced malignancies, its function may change and it may lead to increased aggressiveness of
cancer through the promotion of the epithelial-to-mesenchymal transformation. - Structural abnormalities associated with oncogenes are presented in and few examples of tumor suppressor genes and tumors in which they are affected.
3. Growth-promoting metabolic alterations: Cancer cells show a distinctive form of cellular metabolic alteration (even in the presence of adequate oxygen) characterized by high levels of glucose uptake (via upregulation of GLUT 1 receptors) and increased conversion of glucose to lactose (fermentation) via the glycolytic pathway.
This is known as the Warburg effect or aerobic glycolysis.
Glycolytic intermediate processes are used by cancer cells for the generation of nucleosides and amino acids that are essential for tumor proliferation and growth.
4. Evasion of cell death (Apoptosis, Autophagy, and Necrosis):
Apoptosis is a programmed cell death and is one of the normal protective mechanisms by which a cell with DNA damage (mutation) undergoes cell death.
Mutations in the genes that regulate apoptosis may lead to neoplasm. Examples
Autophagy is a natural process that allows cells to break down intracellular organelles upon exposure to stressors with the help of lysosomes and recycle nutrients.
It is also a protective process in case of neoplastic transformation.
Cancer cells may use autophagy to recycle their nutrients and escape damaging agents.
This process is regulated by PI3-kinase, AKT, and mTOR pathway and by the protein Beclin-
Necrosis is another process of cell death in which cells increase in size and break, releasing multiple proinflammatory cytokines.
Immune cells are attracted to the areas of necrosis to eliminate the remnants of cells.
Cancer can use this process to induce an inflammatory preneoplastic environment, stimulate angiogenesis, and even use these cytokines to stimulate its own growth.
5. Limitless replicative potential: All malignant tumors contain cells that are immortal and have limitless replicative potential.
- Cancer stem cells: At least a few cells in all cancers have stem cell-like properties and are called cancer stem cells.
- Reactivation of telomerase: During the course of repeated cell cycles, there is progressive shortening of telomeres, making the cells vulnerable to senescence/apoptosis.
- The cells that are able to maintain the activity of telomerase, an enzyme that is responsible for the lengthening of telomeres, can potentially proliferate uncontrollably and turn into
cancer.
6. Development of sustained angiogenesis: Microscopic lesions rely on osmosis for the delivery of nutrients, but it has been shown that early in carcinogenesis, the “angiogenic switch” occurs that promotes new blood vessel formation.
Vascular endothelial growth factor A (VEGF-A) is the main protein responsible for neoangiogenesis. Its action is counterbalanced by the activity of thrombospondin-1 (TSP-1).
7. Ability to invade and metastasize (invasion and metastasis): Cancer cells are characterized by a unique ability of local invasion and formation of distant metastasis.
E-cadherin is one of the most important cell surface adhesion molecules responsible for maintaining tissue integrity.
Multiple cancers express E-cadherin at a low level and express molecules implicated in cell migration such as N-cadherin at higher levels.
Formation of distant metastasis is a multi-step process and it consists of the following:
- Local invasion
- Migration into lymphatic and blood vessels
- Spread of cancer cells through the vasculature
- Extravasation of cancer cells into the tissue of remote organs
- Growth of cancer cells in the new environment to form macroscopic tumors
Molecular events occurring during the process of formation metastasis resemble steps of embryonic morphogenesis.
The process by itself has been named epithelial-mesenchymal transition (EMT) and involves genes playing a physiologic role in embryogenesis such as Snail, Slug, Twist, and Zeb1/2.
These genes are regulated by intracellular oncogenic events, but they can be also influenced by microenvironmental stimuli.
8. Ability to evade the host immune response: The immune cells eliminate pathogens and potentially cancerous cells.
Cancer cells evade this surveillance by disabling components of the immune system.
Genetic Basis Of Transformation Of A Normal Cell Into A Malignant Cell
Question 3. Describe the genetic basis of selected cancers.
Answer:
Molecular Basis of Cancer
Nonlethal genetic damage lies at the heart of carcinogenesis. Such genetic damage (or mutation) may be acquired by the action of environmental agents, such as chemicals, radiation, or viruses, or it may be inherited in the germ line.

- A tumor is formed by the clonal expansion of a single precursor cell that has incurred genetic damage.
- Four classes of normal regulatory genes—the growth-promoting proto-oncogenes, the growth-inhibiting tumor suppressor genes, genes that regulate programmed cell death (apoptosis), and genes involved in DNA repair—are the principal targets of genetic damage.
- Carcinogenesis is a multistep process at both the phenotypic and the genetic levels, resulting from the accumulation of multiple mutations.
Kinetics of tumor growth: Tumor growth depends on the size of the proliferating pool of cells and the number of cells dying spontaneously. The larger the tumor mass, the greater the percentage of nondividing and dying cells and the longer it takes for the average cell to divide.

Genomic Stability
The mechanisms responsible for genome stability (repair mechanisms) assure that any randomly created mutations are repaired or an abnormal cell is eliminated.
In the presence of external mutagens or heritable susceptibility factors such as mutations in tumor suppressor genes, the repair mechanisms malfunction and allow abnormal cells to survive. The genes involved are caretaker genes and gatekeeper genes as described below.

Tumor-promoting Inflammation
Tumors consist not only of neoplastic cells but also of a variety of cells of the innate and adaptive immune systems.
The density of immune cells and their localization within the tumor are different among different malignancies.
Initially, it was postulated that the presence of these cells reflected an attempt to eradicate a tumor by the immune system.
Currently, we know that these inflammatory infiltrates can actually promote tumorigenesis and cancer growth by providing cytokines and growth and survival factors for the tumor, by stimulating angiogenesis, and by facilitating invasion and metastasis.
Inflammation can ignite the transition from a premalignant state to full malignancy, for example, by the release of reactive oxygen species that have mutagenic capabilities.
Question 4. What are the differences between benign and malignant tumors?
Answer:

Question 5. Write a short note on metaplasia, dysplasia, and anaplasia.
Answer:

Common Cancers
Question 6. Describe the clinical epidemiology and inherited and modifiable risk factors for common malignancies in India.
Answer:

Risk Factors of Cancer

Etiology of Cancer
Question 7. Write a note on the etiological factors of cancer—genetic, chemical, and infectious agents.
Answer:
Genetic Predisposition

Chemical Carcinogenesis
It is a two-step process.
Chemical Carcinogenesis Initiation: Initiation results from the exposure of cells to a sufficient dose of a carcinogenic agent (initiator). Initiation alone, however, is not sufficient for tumor formation.
Initiation causes permanent DNA damage (mutations). It is therefore rapid and irreversible and has “memory”.
It can be two types of agents (directly acting and indirectly acting agents).
Chemical Carcinogenesis Promotion: Promoters can induce tumors in initiated cells, but they are nontumorigenic by themselves.
The cellular changes resulting from the application of promoters do not affect DNA directly and are reversible.
Promoters enhance the proliferation of initiated cells, an effect that may contribute to the development of additional mutations in these cells.

Question 8. Write a short note on the Ames test. Test for mutagenicity (Ames test):
Answer: It is a biological assay to assess the mutagenic potential of chemical compounds.
A positive test indicates that the chemical is mutagenic and therefore may act as a carcinogen.
The Ames test uses several strains of the bacterium Salmonella typhimurium that carry mutations in genes involved in histidine synthesis.
These strains are auxotrophic mutants, i.e., they require histidine for growth, but cannot produce it.
The method tests the capability of the tested substance in creating mutations that result in a return to a “prototrophic” state so that the cells can grow on a histidine-free medium.
Infection and Cancer
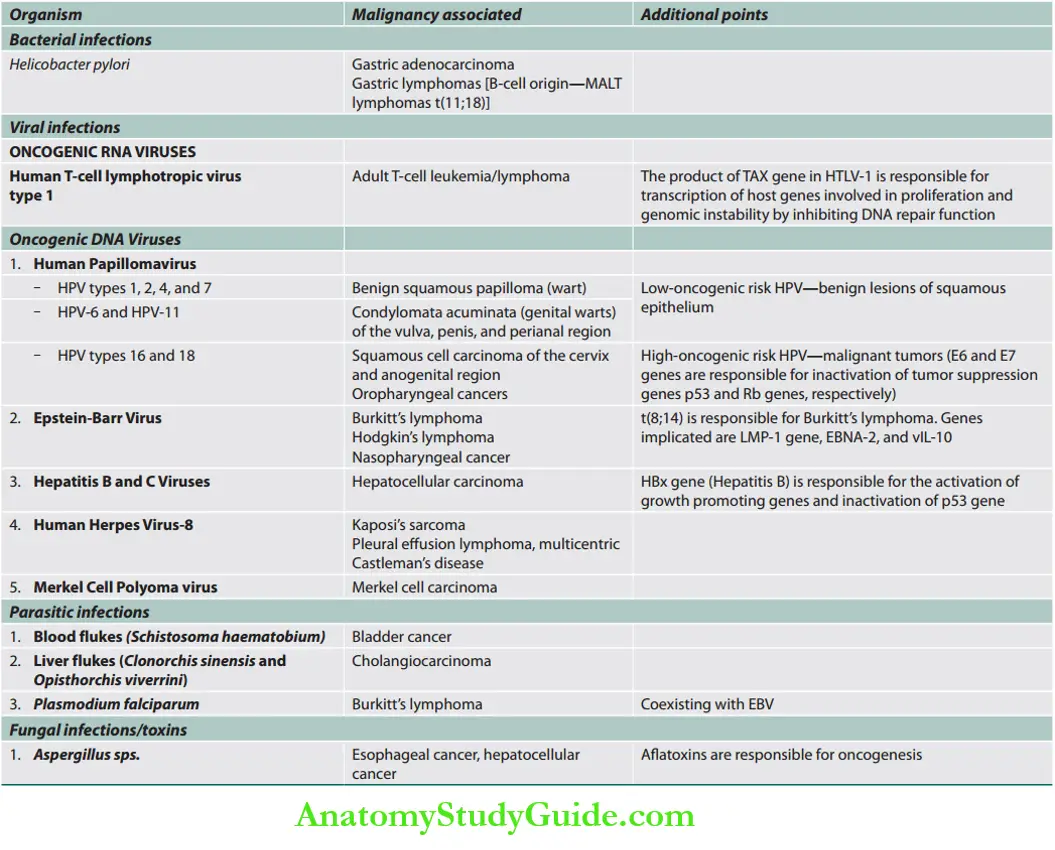
Cancer Screening And Prevention
Question 9. Describe the relationship between infection and cancers. (or) Describe the role of oncogenic viruses in the evolution of virus-associated malignancy. Write a note on cancer screening and prevention.(or) Describe the need, tests involved, and their utility in the prevention of common malignancies.
Answer:
Investigations


Diagnosis And Staging Of Cancer
Question 10. Enumerate the list of investigations useful in diagnosis, staging, and monitoring response to treatment in a cancer patient.
(or)
Order and interpret diagnostic testing based on the clinical diagnosis including CBC and stool occult blood, and prostate-specific antigen.
Answer:




Tumor Markers

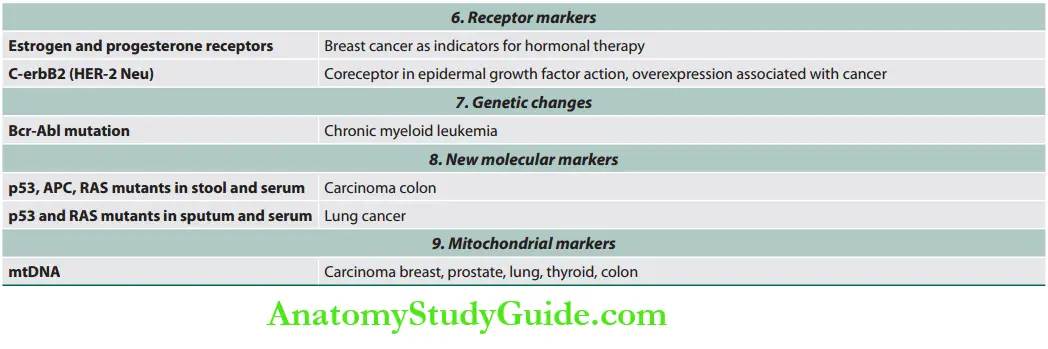
Staging Of Cancer
Question 11. Enumerate, describe, and discuss the classification and staging of cancer (AJCC, FIGO, etc.).
Answer:
Staging Of Cancer Staging System
Cancer is often staged twice. The first rating is done before treatment and is called the clinical stage. The second rating is done after treatment, such as surgery, and is called the pathologic stage.
The pathologic stage is more precise than the extent of the cancer.
Staging Of Cancer TNM Staging System
- The TNM staging system is the most common method used for cancer staging.
- It is maintained by AJCC (American Joint Committee on Cancer) and UICC (Union for International Cancer Control).
- In this system, the letters T, N, and M describe a different area of cancer growth.
- Not all cancers are rated by TNM scores (exceptions include hematological cancers)

Staging Of Cancer Stage Groups
- Each type of cancer has its own stage groups based on where the cancer has grown and spread. There are either four or five stage groups per cancer (ranked by Roman numerals starting with either stage 0 or stage I and ending at stage IV).
- Stage groups for most cancers are defined by TNM scores. If TNM scores are not used, stage groups are still defined by where the cancer is in the body.
- Some cancers that are grouped by TNM scores also use other information to define the stage groups.
- All people who meet the criteria of a stage group have a similar prognosis.
- In general, cancers that cannot spread to distant sites are rated as stage 0. Stage I includes small primary tumors that have not spread to lymph nodes.
- Stage II and III are larger or more extensive primary tumors with or without cancer in nearby lymph nodes. Stage IV is cancer that has spread to distant sites at diagnosis.
FIGO Staging System

It is determined by the International Federation of Gynecology and Obstetrics and used for gynecological cancers. In general, there are five stages.
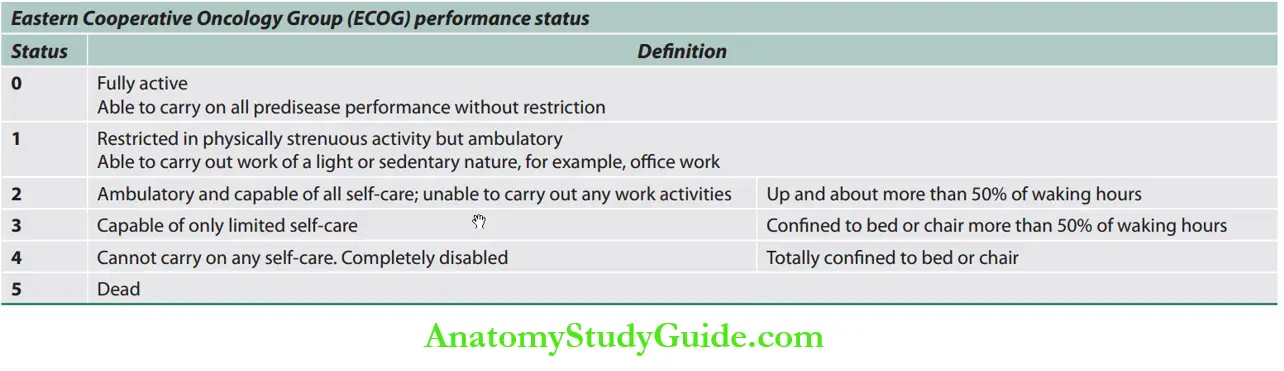
Performance Status
Performance status is a measure of a patient’s functional capacity.
It has consistently been found to predict survival in patients with cancer, and it is used in that capacity as an entry criterion and an adjustment factor for clinical trials of anticancer therapies.
A number of metrics have been developed to quantify performance status; among them, the Eastern Cooperative Oncology Group (ECOG) performance status and Karnofsky Performance Status (KPS) are the most commonly used.

Cancer Treatment
Management of Cancer Patient
Question 12. Describe and distinguish the difference between curative, palliative, and hospice care in patients with cancer.
(or)
Demonstrate an understanding of and needs and preferences of patients when choosing curative and palliative therapy.|
(or)
Describe the therapies used in alleviating suffering in patients at the end of life (palliative therapy and hospice).
(or)
Write a short note on adjuvant and neoadjuvant chemotherapy.
Answer:

Precision medicine (also known as personalized medicine) is an emerging approach to disease treatment and prevention that takes into account individual variability in genes, environment, and lifestyle for each person.




Chemotherapeutic Agents
Question 13. Write a short note on the classification of chemotherapeutic agents.
(or)
Write a short note on cyclophosphamide.
(or)
Write a short note on cisplatin.
(or)
Write a short note on methotrexate.
Answer:












Targeted Therapy
Question 14. Write a short essay/note on various types of targeted therapy in cancer patients.
Answer:



The currently approved conjugated antibodies have been
Question 15. Write a short note on immunosuppressive drugs.
Answer: These drugs inhibit cellular and/or humoral immunity response and have a major role in organ transplantation and autoimmune diseases.
A list of immunosuppressive drugs useful in cancer management is enumerated
Conjugated antibodies.
Conjugated antibodies currently approved
- Radio-conjugated antibodies
- Tositumomab
- Ibritumomab
- Both used against refractory lymphomas
- Toxin-conjugated antibody
- Gemtuzumab ozogamicin
- Used against AML
Immunosuppressive drugs.
Immunosuppressive drugs
- Calcineurin inhibitors (specific R cell inhibitors): For example, cyclosporine, tacrolimus
- Antiproliferative drugs (cytotoxic drugs): Azathioprine, cyclophosphamide, methotrexate, chlorambucil, mycophenolate mofetil (MMF)
- Glucocorticoids: Prednisolone and others
- Antibodies: Muromonab CD3, anti-thymocyte globulin (ATG), Rho (D)
immunoglobulin
Pain Assessment And Management
Question 16. Describe and assess pain and suffering objectively in a patient with cancer.(or) Describe and enumerate the indications, use, and side effects of narcotics in pain alleviation in patients with cancer.
Answer:
Assessment of Pain
- Careful pain history (site of pain, onset, acute or chronic, constant or intermittent, provoking and alleviating factors, and associated symptoms)
- Quality of the pain (nociceptive somatic, nociceptive visceral, and neuropathic pain)
- Impact on daily activities
- The pain interventions already tried and their level of effectiveness
- Screen for a history of alcohol or drug dependence
- Evaluation for depression
- Physical examination.
Scaling of pain (Wong-Baker FACES pain rating scale).

Cancer Pain Management
Pain is a highly prevalent symptom in patients with cancer. Adequate pain relief can be achieved in 90% of patients when treatment guidelines for cancer pain are followed.
Common Side Effects of Narcotics (Opioids)
- Gastrointestinal: Constipation, nausea, and vomiting
- CNS: Sedation, confusion, and myoclonus
- Urinary retention
- Tolerance
- Drug addiction and abuse
Oncologic Emergencies
Question 17. Write a short note on:
Answer:
Emergency conditions related to tumors.
Clinical features and treatment of tumor lysis syndrome.
Tumor Lysis Syndrome (TLS)
Tumor lysis syndrome (TLS) is a treatment-related complication. It can develop 1–5 days after postchemotherapy treatment of leukemias and lymphomas.
- Metabolic triad hyperuricemia, hyperkalemia, hyperphosphatemia.
- It can also lead to renal failure and hypocalcemia as secondary complications.
- It is due to the release of the breakdown products of dying tumor cells.
- Chemotherapeutic agents cause cell lysis and cell death with the release of intracellular components into the bloodstream.
Breakdown of nucleic acid, catabolism of hypoxanthine and xanthine lead to elevated uric acid.
Potassium and phosphate are present at high levels in the cytoplasm.
Risk factors are Large tumor burden, high growth fraction, and increased pretreatment.
LDH or uric acid, or preexisting renal insufficiency.

Oncologic Emergencies Clinical Features
Lysis of malignant cells causes several metabolic abnormalities/disturbances.
These include hyperkalemia (leads to cardiac arrhythmias), hyperphosphatemia (produces acute renal failure), hyperuricemia (produces acute renal failure), hyperuricosuria, hypocalcemia (results in seizures, muscle cramps, tetany, and arrhythmia), and consequent acute uric acid nephropathy and renal failure.
Oncologic Emergencies Prophylaxis
Patients at high risk: Leukemia, high-grade lymphoma, rapidly proliferating bulky solid tumor (e.g., small cell) must receive vigorous hydration, allopurinol, and careful metabolic monitoring.
Treatment: Intravenous hydration is of prime importance.
Oncologic Emergencies Rasburicase (works for prevention and treatment)
-
- Contraindicated in G6PD deficiency.
- Rasburicase (recombinant urate oxidase) converts uric acid into allantoin, which is an inactive and soluble metabolite and is easily excreted by the kidneys.
- Alkalinization of urine to promote uric acid excretion is controversial (it may worsen hypocalcemia tetany).
- Allopurinol can be used to decrease uric acid.
- Management of life-threatening hyperkalemia with anti-hyperkalemic measures.
- For hypocalcemia, infuse calcium gluconate under ECG monitoring.
- Hyperphosphatemia can be treated by giving aluminum hydroxide
orally or on hemodialysis.
Early dialysis (hemodialysis is preferred) is required when uric acid >10 mg/dL, phosphorus >10 mg/dL, or creatinine >10 mg/dL.
Febrile Neutropenia/Neutropenic Sepsis/Neutropenic Fever
Question 18. Write a short essay/note on febrile neutropenia.
Answer:
Neutropenia (decrease of neutrophils below normal range) is a common complication of malignancy.
It can occur secondary to chemotherapy, radiotherapy (if large amounts of bone marrow are irradiated), or as a component of pancytopenia due to infiltration of the bone marrow by malignancy.
Neutropenic fever is defined as a fever/pyrexia of 38.3°C for >1 hour in a patient with a neutrophil count <500/mm3.
The risk of sepsis is dependent on the severity and duration of neutropenia and the presence of other risk factors (e.g., intravenous or bladder catheters).
febrile neutropenia Clinical Features
The typical presentation is with a high fever. Neutropenic patients are prone to bacterial and fungal infection, most often from endogenous sources (e.g., enteric bowel flora).
Carefully examine for potential foci of infection. However, the examination usually does not define the primary source of the infection.
Signs and symptoms of infection may be minimal, particularly in patients receiving corticosteroids.
Apart from fever, patients may also have nonspecific symptoms (e.g., nausea, diarrhea, drowsiness, and breathlessness).
Hypotension is a bad prognostic feature and may lead to systemic circulatory shutdown and organ failure.
febrile neutropenia Investigations
Full blood count, cultures (urine, sputum, stool depending on the case), chest X-ray, and swabs for culture (throat, central line, wound).
Before antibiotic therapy, two sets of blood cultures from a peripheral vein and any indwelling venous catheters should be sent.
febrile neutropenia Treatment
Upon initial evaluation, each patient should be assessed for risk of complications from severe infection.
Appropriate risk assessment may determine the type of empiric therapy (oral vs IV), duration of antibiotic therapy, and determination of inpatient versus outpatient management.
Patients are classified into high-risk and low-risk groups.
Low-risk group High-risk group
- Anticipated brief (<7-day duration) period of neutropenia
- ANC > 100/µL and absolute monocyte count > 100/µL
- Normal findings on chest radiograph
- Outpatient status at the time of fever onset
- No associated acute comorbid illness
- No hepatic or renal insufficiency
- Early evidence of bone marrow recovery
Anticipated, prolonged (>7-day duration), and profound neutropenia (ANC < 100/µL) following cytotoxic chemotherapy Significant medical comorbidities, including hypotension,
pneumonia, new-onset abdominal pain, or neurologic changes.
febrile neutropenia Antibiotics

febrile neutropenia Antifungal: If there is no response to antibiotics after 72–96 hours, treatment with amphotericin B or voriconazole should be given to cover fungal infection.
febrile neutropenia Growth factors: G-CSF and GM-CSF are given to improve blood counts.
febrile neutropenia Other supportive therapy: Patients with signs of systemic illness, such as tachycardia, hypotension, and oliguria require urgent admission and resuscitation with intravenous fluids to restore circulatory function.
Other supportive therapy includes inotrope therapy, ventilation, or hemofiltration.
Febrile neutropenia Prophylactic floroquinolones
It is given to high-risk and intermediate-risk groups (consisting of patients receiving high-dose chemotherapy and those with hematological malignancy) in which the anticipated duration of neutropenia is >7 days.
Prophylactic fluoroquinolones are not indicated in patients with solid tumors undergoing standard outpatient cyclical chemotherapy in which the anticipated duration of neutropenia is <7 days, because it may lead to microbial resistance.
Paraneoplastic Syndromes
Question 19. Write a short essay/note on paraneoplastic syndromes.
Answer:
Paraneoplastic Syndromes Definition: Paraneoplastic syndromes are symptom complexes in cancer patients that are not directly related to mass effects or invasion or metastasis or by the secretion of hormones indigenous to the tissue of origin.
Paraneoplastic Syndromes Frequency: Though they occur in 10–15% of patients, it is important because:
Maybe the first manifestation of an occult neoplasm.
May be mistaken for metastatic disease leading to inappropriate treatment.
May present clinical problems which may be fatal.
Certain tumor products causing paraneoplastic syndromes may be useful in monitoring recurrence in patients who had surgical resections or undergoing chemotherapy or radiation therapy.
Some paraneoplastic syndromes, their mechanism, and common cancer-causing them are listed





Leave a Reply