RNA Oncogenic Viruses
RNA oncogenic viruses are retroviruses i.e. they contain the enzyme reverse transcriptase (RT), though all retroviruses are not oncogenic.
Table of Contents
The enzyme, reverse transcriptase, is required for the reverse transcription of viral RNA to synthesize viral DNA strands i.e. the reverse of normal—rather than DNA encoding for RNA synthesis, viral RNA transcripts for the DNA by the enzyme RT present in the RNA viruses.
Read And Learn More Neoplasia
RT is a DNA polymerase that helps to form complementary DNA (cDNA) that moves into the host cell nucleus and gets incorporated into it.
Based on their activity to transform target cells into neoplastic cells, RNA viruses are divided into 3 subgroups
- Acute transforming viruses
- Slow-transforming viruses and
- Human
T-cell lymphotropic viruses (HTLV). The former two are implicated in inducing a variety of tumors in animals while HTLV is involved in the etiology of human T-cell leukemia and lymphoma.
Acute-Transforming Viruses
This group includes retroviruses which transform all the cells infected by them into malignant cells rapidly (‘acute’).
- All the viruses in this group possess one or more viral oncogenes (v-once). All the members of acute transforming viruses discovered so far are defective viruses in which the particular v-onc has substituted other essential genetic material such as gag, pol, and env.
- These defective viruses cannot replicate by themselves unless the host cell is infected by another ‘helper virus’.
Acute oncogenic viruses have been identified in tumors in different animals only for example,
- Rous sarcoma virus in chickens.
- Leukaemia-sarcoma viruses of various types such as avian, feline, bovine, and primate.
Slow-Transforming Viruses
These oncogenic retroviruses cause the development of leukemias and lymphomas in different species of animals (for example, Mice, cats, and bovine) and include the mouse mammary tumor virus (MMTV) that causes breast cancer in the daughter mice suckled by the MMTV-infected mother via the causal agent in the mother’s milk (Bittner milk factor).
- These viruses have a long incubation period between infection and the development of neoplastic transformation (‘slow’).
- Slow-transforming viruses cause neoplastic transformation by insertional mutagenesis i.e. viral DNA synthesised by viral RNA via reverse transcriptase is inserted or integrated near the proto-oncogenes of the host cell resulting in mutational damage to the host cell genome leading to neoplastic transformation.
Human T-cell lymphotropic Viruses (HTLV)
Htlv is a form of the slow-transforming virus but is described separately for 2 reasons:
- This is the only retrovirus implicated in human cancer.
- The mechanism of neoplastic transformation is different from slow-transforming as well as from acute-transforming viruses.
Four types of HTLVs are recognised—HTLV-1, HTLV-2, HTLV-3 and HTLV-4:
-
- It may be recalled here that HIV, the etiologic agent for AIDS, is also an HTLV (HTLV-3).
- HTLV-2 is implicated in the etiology of the T-cell variant of hairy-cell leukemia.
- A link between HTLV-1 infection and cutaneous adult T-cell leukaemia-lymphoma (ATLL) has been identified.
- HTLV-1 is transmitted through sexual contact, by blood, or to infants during breastfeeding.
The highlights of this association are as under:
- Epidemiological studies by tests for antibodies have shown that HTLV-1 infection is endemic in parts of Japan and the West Indies where the incidence of ATLL is high. The latent period after
- HTLV-1 infection is, however, very long (20–30 years). The initiation of the neoplastic process is similar to that for Burkitt’s lymphoma except that
- HTLV-1 has a tropism for CD4+T lymphocytes as in HIV infection, while EBV of Burkitt’s lymphoma has a tropism for B lymphocytes.
- As in Burkitt’s lymphoma, immunosuppression plays a supportive role in the neoplastic transformation by HTLV-1 infection.
HTLV-1 Oncogenesis
The molecular mechanism of ATLL leukemogenesis by HTLV-1 infection of CD4+ T lymphocytes is not clear.
Neoplastic transformation by HTLV-I infection differs from acute-transforming viruses because it does not contain v-onc, and from other slow-transforming viruses because it does not have a consistent site of insertion of mutations, nor does it contain an oncogene. Probably, the process is multifactorial:
RNA oncogenic viruses:
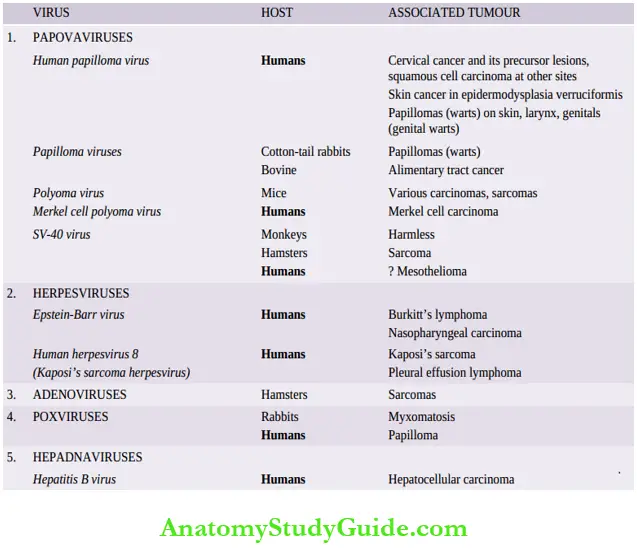
- HTLV-1 genome has a unique region called pX distinct from other retroviruses, which encodes for two essential viral oncoproteins—TAX and REX.
- TAX protein up-regulates the expression of cellular genes controlling T-cell replication, while REX gene product regulates viral protein production by affecting mRNA expression.
- TAX viral protein interacts with transcription factor, NF-κB, which stimulates genes for cytokines (interleukins) and their receptors in infected T-cells which activates proliferation of T cells by autocrine pathway.
- The inappropriate gene expression activates the pathway of cell proliferation by activation of cyclins and inactivation of tumor-suppressor genes CDKN2A/p16 and p53, stimulating the cell cycle.
- Initially, a proliferation of infected T-cells is polyclonal but subsequently, several mutations appear due to TAX-based genomic changes in the host cell and monoclonal proliferation of leukemia occurs.
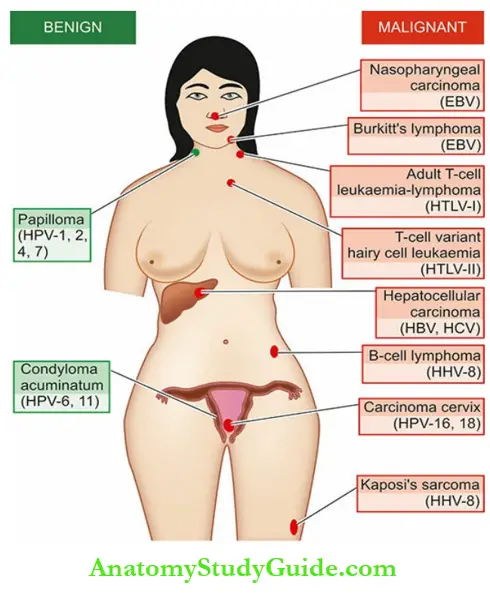
Salient contrasting features of DNA and RNA oncogenic viruses are summarised in the given Examples of common human tumors in which oncogenic viruses are implicated are shown.
- Our current knowledge and understanding of viral carcinogenesis have provided an opportunity to invent specific vaccines and suggest appropriate specific therapies.
- For example, the hepatitis B vaccine is being widely used to control hepatitis B to lower the incidence of HBV-related hepatocellular carcinoma in high-risk populations.
- Similarly, the HPV vaccine is being used in some countries by young women to protect them against HPV-associated precancerous lesions of the cervix.
Carcinogens and Carcinogenesis:
Important groups of carcinogenic agents having a role in carcinogenesis are chemical, physical, and biological agents.
Chemical carcinogenesis occurs by induction of mutation in the proto-oncogenes and tumor-suppressor genes and goes through sequential stages of initiation, promotion, and progression.
Carcinogenic chemicals are of 2 types:
- Direct-acting and
- Indirect-acting.
These are
- Direct-acting carcinogens (for example, alkylating agents, and alkylating agents) can induce cellular transformation without undergoing any prior metabolic activation.
- Indirect-acting carcinogens (for example, Polycyclic aromatic hydrocarbons, aromatic amines, azo dyes, naturally occurring products, etc) require metabolic activation within the body to become ‘ultimate’ carcinogens.
Physical agents in carcinogenesis are radiation (ultraviolet light and ionizing radiation) which is more important, and some non-radiation physical agents.
- Excessive exposure to UV rays in humans can cause various forms of skin cancers while ionizing radiation is implicated in cancers of different organs for example, Leukaemias, Cancers of the Thyroid, Skin, Breast, Ovary, Uterus, Lung, Myeloma, and Salivary glands.
- Out of biological agents (viruses, bacteria, parasites, fungi), the persistence of DNA or RNA viral infection is of major significance and may induce mutation in the target host cell which is one step in the multistep process of cancer development.
- In addition, H. pylori is involved in gastric lymphoma and gastric carcinoma.
- RNA viruses having oncogenic roles are HTLV-1 (adult T-cell leukemia and lymphoma), HTLV-2 (T-cell variant of hairy cell leukemia), and hepatitis C virus (hepatocellular carcinoma).
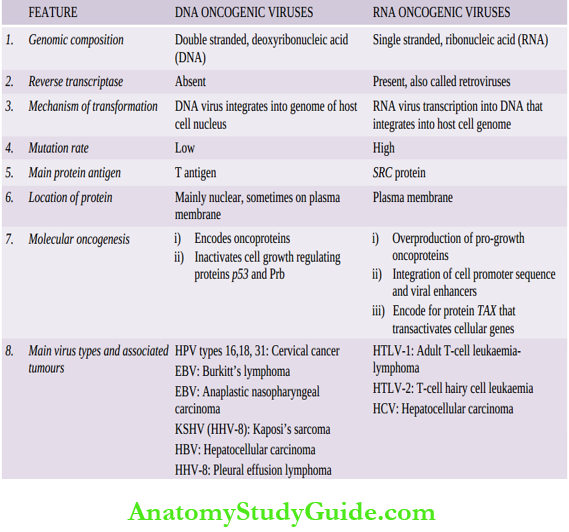
Salient contrasting features of DNA and RNA oncogenic viruses:
Leave a Reply