Antibacterial Sulphonamides Introduction
Antibacterial sulphonamides are first effective chemotherapeutic agents used for bacterial infection in humans. The term sulphonamide is usually employed as a generic name for the derivatives of p-amino benzene sulphonamides. The compound p-amino benzene sulphonamide was first synthesized by Gelmo in 1908 as an intermediate in the study of azo dyes. Work on azo dyes containing a sulphonamide nucleus has been started by research workers of the I.G.
Table of Contents
Farbenindustrie about 1930. In 1932, Gerhard Domagk recognized the antibacterial activity of the azo dye, Prontosil red. It was effective in the treatment of hemolytic Streptococcal infection. Prontosil is totally inactive in vitro but possess excellent activity in vivo. In 1935, Trofouel at Pasteur Institute discovered that Prontosil breaks down in the tissues into p-amino benzene sulphonamide and suggested that antibacterial characteristic of the drug resides in this part of the molecule.
This discovery leads to the synthesis of nearly 5500 various sulphonamides, but only few are in clinical practice. The rapid development of widespread resistance to the sulphonamides and the increasing use of the broad spectrum Penicillin’s in the treatment of infectious disease diminished the usefulness of sulphonamides. The development of combination of Trimethoprim and Sulphonamide in the treatment and prophylaxis on certain opportunistic microbial infection, reawaked interest in sulphonamides.
Sulphonamides inhibit both Gram-positive and Gram-negative bacteria, Nocardia, Chlamydia trachomatis and some Protozoa. Some enteric bacteria such as E.coli, Kelbsiella, Salmonella, Shigella and Enterobacter are inhibited. Sulphonamides are used in the treatment of tonsillitis, septicemia, meningococcal meningitis, bacillary dysentery and number of infections of urinary tract.
Nomenclature of Sulphonamides
Sulphonamides are considered as derivatives of p-amino benzene sulphonamide. The nitrogen atom of – SO2NH2 is numbered as 1 and the -NH2 group as 4.
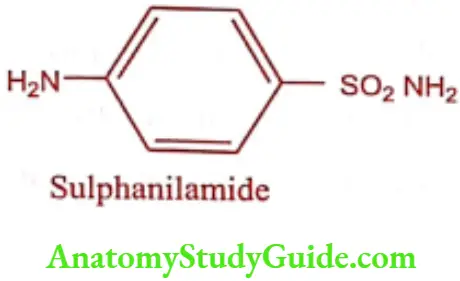
Sulphonamides General Properties
Sulphonamides are white crystalline powder, sparingly soluble in water. The sulphonamide group SO2NH2 loses a proton and form salts, which is more soluble than free sulphonamides. The negative charge on nitrogen, after the loss of proton, gain stability by resonance.
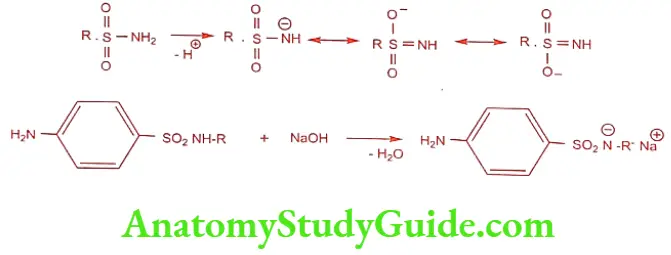
Crystalluria and the pKa
Sulphonamides and their metabolites are excreted almost in the urine. Sulphonamides are not water soluble; they crystallize in the kidney and causes crystalluria. The pKa of sulphamido group of Sulphanilamide is 10.4, that the pH at which 50% of the drug is ionized. Because the urine pH is 6, essentially, all the sulphonamides are unionized and occur as insoluble form in the kidney. The sulphonamide coming out of solution in urine and kidney causes crystalluria.
Various approaches to adjust the solubility of sulphonamide in urine were performed. They are:
- Greatly increasing the urine flow by taking plenty of fluids, so that glomerular filtration rate could be increased, there would be less opportunity to form crystals in renal tubules.
- Increasing the pH of the urine: This is achieved by giving oral sodium bicarbonate before to sulphonamide dose, so that the urine pH will be closure to 10.4.
- Mixed sulphonamides: Mixing different sulphonamide to achieve an appropriate total dose. When several sulphonamides are administered together, the antibacterial activity is the summation of the total sulphonamide concentration present, but the solubility is independent of each other. Thus by giving a mixture of sulphonamide the same therapeutic level can be maintained with much less danger of crystalluria, because only one-third of the amount of any one compound is present. Hence, mixed sulphonamides are in current practice now. For example,
- Trisulphapyrimidine contains equal amount of Sulphadiazine, Sulphamerazine and Sulphamethazine. It is available as oral suspension and tablets.
- Sulphadoxine and Pyrimethamine: The mixture of Sulphadoxine and Pyrimethamine is used for the treatment of Plasmodium falciparum malaria.
- A newer sulphonamide has been synthesized whose pKa is closer to pH of urine. This is brought about, by introducing an electron withdrawing, hetero cyclic ring at N1 position, which donates a proton and lowers the pKa value.
Sulphonamides Mechanism of Action
Sulphonamides are bacteriostatic in nature. The sulphonamide sensitive microorganisms require p-Amino benzoic acid (PABA) for the synthesis of folic acid which is essential for the synthesis of DNA and RNA. Various folate derivatives like folinic acid, N3, N1o-methylene tetrahydro folic acid and N10-formyl tetrahydro folic acid acts as coenzymes in transport of one carbon unit in several biochemical reactions in humans and micro organisms.
The one carbon transfer reaction is necessary for the synthesis of purines, thymidine and some amino acids. One of the products of this folate dependent biochemical reaction is deoxy thymidine mono phosphate, which is an important precursor to DNA. Sulphonamides block the biosynthesis of this folate coenzyme resulting into the arrest of bacterial growth and cell division.
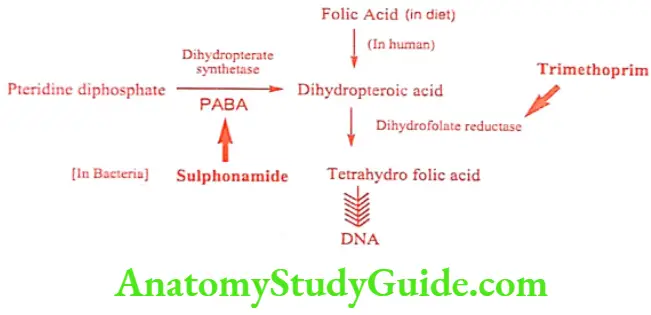
The sulphonamides are structural analogues to PABA that competitively inhibit the action of dihydropteroate synthetase, preventing the addition of PABA to pteridine diphosphate and blocking the net biosynthesis of folate coenzymes.
Folate coenzymes are biosynthesized from dietary folic acid in human and other cells. Bacteria and protozoa must biosynthesis it de novo from PABA and pteridine diphosphate. Microbes cannot take up the folic acid from the growth medium or from the host (bacterial cell wall may be impermeable to folic acid). Hence sulphonamides does not affect mammalian cell.
The conversion of dihydrofolic acid to tetrahydrofolic acid is catalyzed by enzyme dihydrofolate reductase. Trimethoprim is a selective competitive inhibitor of microbial dihydrofolate reductase. So, when combined with sulpha drug, it appears to be most active agent that exerts a synergistic antibacterial activity. Trimethoprim does not have high affinity for malarial protozoan’s folate reductase. In antimalarial therapy, Pyrimethamine and sulpha combination yields synergistic effect.
Sulphonamides Structure Activity Relationship
- The free amino group (-NH2) is essential for activity. However it can be replaced by groups which can be reconverted it back to free amino group eg. succinyl amido, acetamido and phthalyl amido group.
- The sulphanilamide skeleton is the minimal structural requirement for antibacterial activity.
- Sulphur should be directly linked to the benzene ring.
- The amino and sulfonyl radical should be 1, 4 position; 1, 2 and 1, 3 isomers are therapeutically less active.
- Any substitution on aromatic ring or replacement of benzene ring by other ring decreases or abolishes the activity.
- Substitution of hetero cyclic aromatic nucleus in N1 position yields highly potent compound.
- N’ disubstitution in general, leads to inactive compound because one hydrogen is essential for ionization.
- Maximum antibacterial activity is exhibited by sulphonamides having pKa values between 6.6 to 7.4.
- The lipid solubility influences the pharmacokinetic and antibacterial activity. In general as the lipid solubility increases, the half life and antibacterial activity (in vitro) also increases.
Therapeutic uses
Sulphonamides may be used systemically, topically or orally for local effects depending upon its solubility. Some uses of sulphonamides are:
- Pneumocystis carinii pneumonia : eg. Trimethoprim and Sulphamethoxazole.
- Cerebral toxoplasmosis : eg. Pyrimethamine and Sulphadiazine.
- Urinary tract infection : eg. Sulphamethizole.
- Nocardiosis : eg. Sulphadiazine, Sulphisoxazole.
- Respiratory tract infection : eg. Sulphalene.
- Dermatitis herpetiformis : eg. Sulphapyrimidine.
- Meningococcal infection : eg. Sulphadiazine.
- Burn therapy : eg. Silver sulphadiazine.
- Conjunctivitis and Superficial ocular infections : eg. Sulphacetamide.
- Traveler’s diarrhea (or) GIT infection: eg. Sulphaguanidine.
- Chloroquine resistant malaria : eg. Sulphadoxime with Pyrimethamine.
- Leprosy : eg. Dapsone.
Sulphonamides Classification
Sulphonamide can be classified in various ways
- On the basis of site of action
- Sulphonamides for general infections: eg. Sulphanilamide, Sulphadiazine, Suphamethoxazole, Sulphadoxine, Sulphapyridine, Sulphamethocine, Sulphathiazole.
- Sulphonamides for intestinal infections: eg. Phthalyl sulphathiazole, Succinyl sulphathiazole, Sulphasalazine.
- Sulphonamides for local infections : eg. Sulphacetamide, Mafenamide, Silver sulphadiazine.
- Sulphonamides for dermatitis : eg. Dapsone, Solapsone.
- Sulphonamides for urinary tract infections: eg. Sulphadiazine, Sulphacetamide, Trimethoprim.
- Sulphonamide Combination : eg. Sulphamethoxazole with Trimethoprim Sulphadiazine with Trimethoprim, Sulphamoxole with Trimethoprim, Sulphadoxime with Pyrimethamine.
- On the basis of pharmacokinetic properties
- Poorly absorbed Sulphonamides: (Locally acting sulphonamides) These agents are poorly absorbed in GIT and mainly used to treat intestinal disease or to reduce luminal bacterial population prior to bowel surgery. eg. Sulphasalazine, Phthalyl sulphathiazole.
- Rapidly absorbed and excreted Sulphonamides (Systemic sulphonamides) eg. Sulphamethoxazole, Sulphisoxazole, Sulphadiazine.
- Topically used Sulphonamides: They are mainly applied in burns, conjunctival sac, otic canal and vagina to treat bacterial infection. eg. Mafenide sodium, Sulphacetamide, Silver sulphadiazine.
- On the basis of chemical classification
- N4 – substituted Sulphonamides (pro drugs): eg. Prontosil, Sulphaguanidine,
- N1- substituted Sulphonamides: eg. Sulphadiazine, Sulphacetamide, Sulphadimidine.
- Both N1 and N4 – substituted Sulphonamides: eg. Succinyl sulphathiazole, Phthalyl sulphathiazole.
- Non-aniline Sulphonamides: eg. Mefenide sodium.
- On the basis of pharmacological activity
- Antibacterial agents: eg. Sulphadiazine, Sulphisoxazole.
- Oral hypoglycemic agent: eg. Tolbutamide.
- Diuretics: eg. Furosemide, Bumetanide, Chlorthalidone.
- Dermatitis: eg. Dapsone.
- On the basis of duration of action
- Ultra long acting Sulphonamides: (half life greater than 50 hours) eg. Sulphasalazine, Sulphacetamide, Sulphalene, Sulphadoxine.
- Long acting acting Sulphonamides: (half life greater than 24 hours) eg. Sulphadimethoxine.
- Intermediate acting Sulphonamides: (half life between 10-24 hours) eg. Sulphamethoxazole.
- Short acting Sulphonamides (half life less than 20 hours) eg. Sulphamethizole, Sulphisoxazole.
Sulphonamides General Method of Synthesis
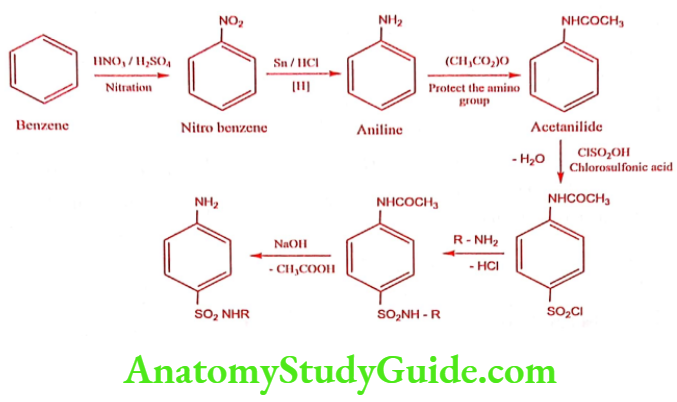
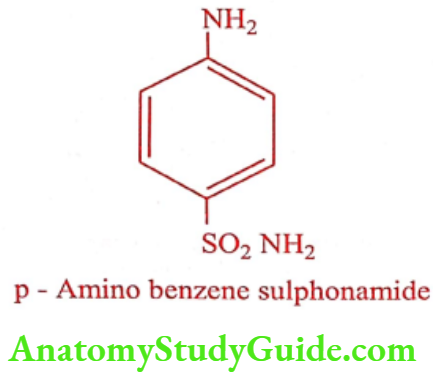
Sulphonamides for General Infections
Sulphanilamide (Prontosil album)
Synthesis
Method – 1
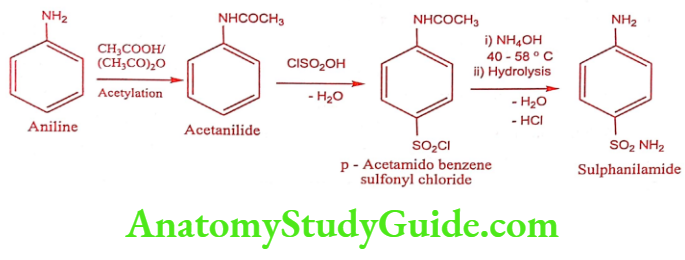
Method – 2
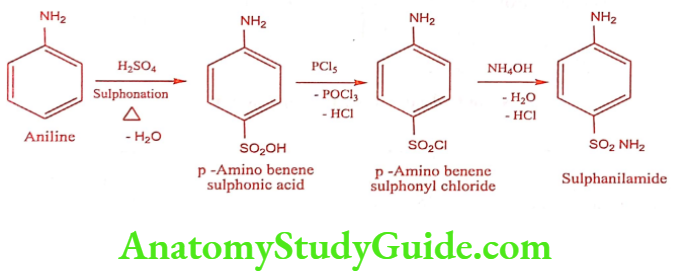
ADR: Severe allergic reactions, difficulty in breathing, fever, persistent sore throat, unusual itching or burning.
Use: Because of its high toxicity it is not in practice now. But still it is used in veterinary practice as antibacterial agent.
Sulphadiazine (Zeotrizine, lantrisul, sulfaloid)
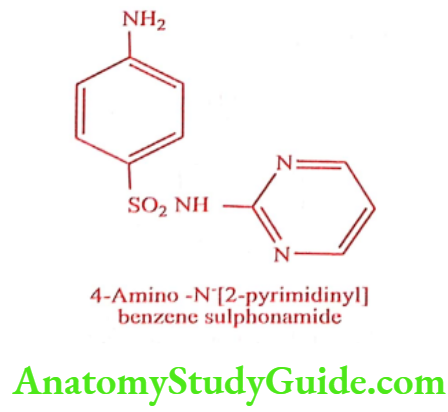
Synthesis
Step 1: Preparation of 2- Amino pyrimidine
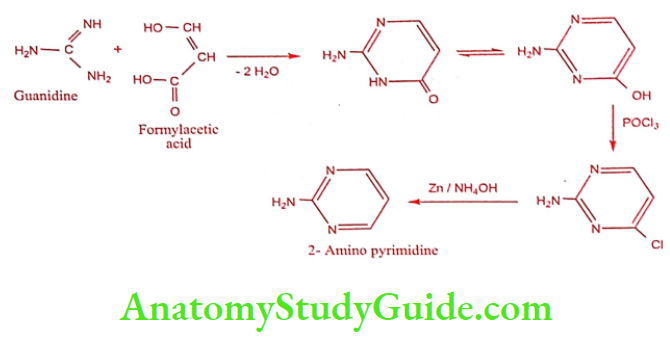
Step 2: Preparation of Sulphadiazine
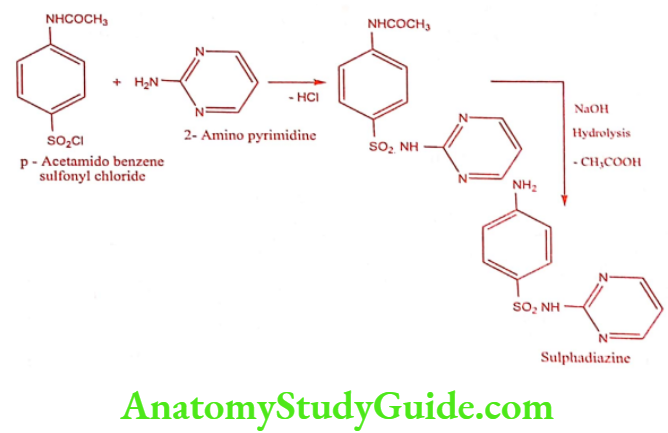
ADR: Nausea, vomiting and anorexia.
Dose: Initially 2 to 4gm/day. May be increased up to 6gm/day in divided doses.
Use: It is used in the treatment of meningitis.
Sulphamethoxazole (Gantanol)
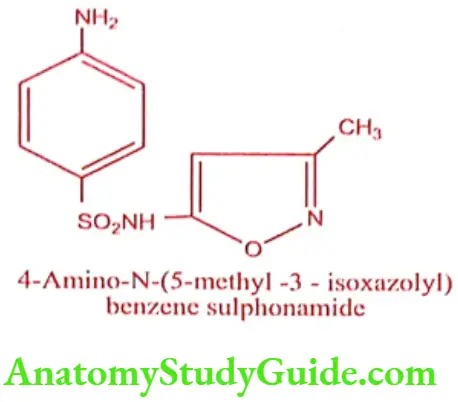
Synthesis
Step 1: Preparation of 3 – Amino 5-methyl isoxazole
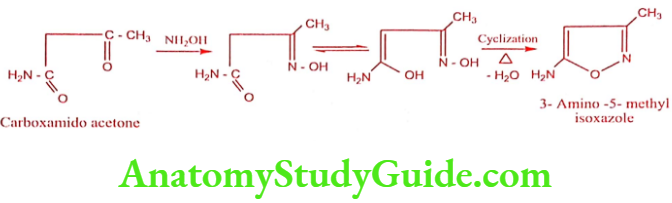
Step 2: Preparation of Sulphamethoxazole
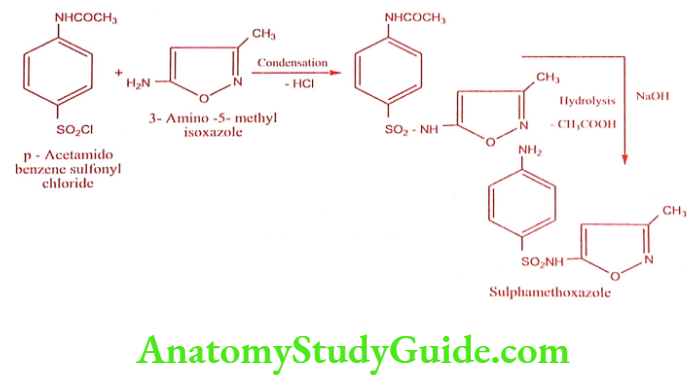
ADR: Nausea, vomiting and epigastric pain.
Dose: 1gm twice daily for 2 days, then 0.5gm twice daily.
Use: It is used in the treatment of meningitis, lower urinary tract infection caused by Escherichia coli and Proteus mirabilis.
Sulphadoxine
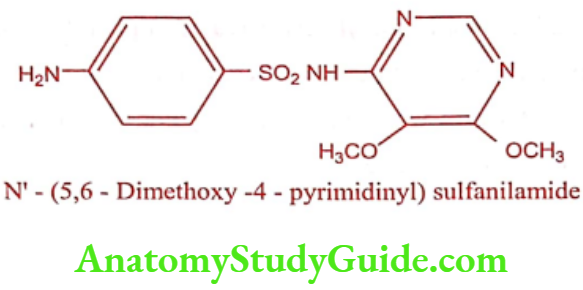
ADR: Urticaria, serum sickness, photosensitization and arthralgia.
Dose: 2 to 3 tablets (1500mg Sulphadoxine and 75mg Pyrimethamine) as a single dose.
Use: Sulphadoxine has antibacterial activity. In combination with Trimethoprim it is used as antibacterial agent with Pyrimethamine and Mefloquine it is used as antimalarial.
Sulphonamides for Intestinal Infections
Sulphasalazine (Saaz, Salazar)
Sulphasalazine is a prodrug, which cleaved at N4 position in large intestine to m-Amino salicyclic acid and Sulphapyridine by azo reductase. Sulphapyridine acts as antibacterial and amino salicylic acid has anti-inflammatory effect on the colon. The advantage of this prodrug is the release of amino salicylic acid, which prevents the absorption of the agent (prevents the systemic absorption) so that duration of action increases (concentrates the drug in active site). It is mainly used in intestinal infections.
ADR: Head ache, anorexia, nausea and vomiting.
Dose: 1to 2gm four times daily.
Use: It is used to treat ulcerative colitis and rheumatoid arthritis.
Sulphonamides for Local Infections
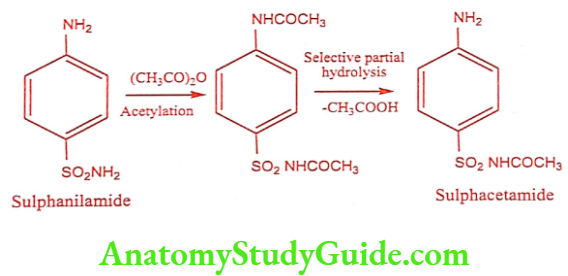
Sulphacetamide (Setride, Zincoren)
Sulphacetamide is a simple acetyl derivative of sulphanilamide, known as Albucid. Sulfacetamide has bacteriostatic effect on a wide range of gram-positive and gram- negative organisms. It interferes with nucleic acid synthesis thus blocking conversion of PABA to the coenzyme dihydrofolic acid.
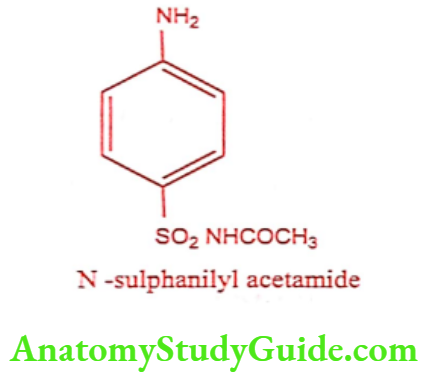
Synthesis
ADR: Hypersensitivity skin reactions, ophthalmic reactions like burning, stinging, corneal ulcers, conjunctivitis, and irritation. Systemic absorption from inflamed conjunctiva. May interfere with diagnostic tests e.g. urinary glucose, urobilinogen, urea and creatinine.
Dose: Apply 10 to 30% as eye drops or 10% ointment into the affected eyes.
Use: Sulphacetamide sodium is used to treat infection or injuries of eye.
Silver sulphadiazine (Silvadene, Silzen)
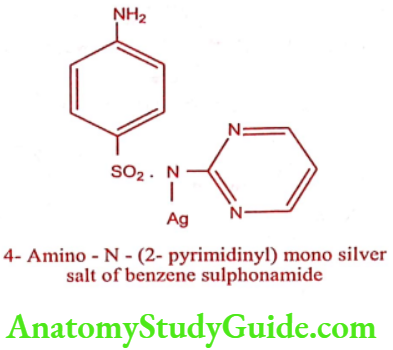
ADR: Nausea, vomiting and diarrhea.
Dose: Apply 1% cream on to affected area.
Use: It is an effective topical antimicrobial agent, especially against Pseudomonas species. It finds extensive use in burn therapy.
Sulphonamide for Dermatitis
Dapsone (DDS)
It is an antibacterial most commonly used in combination with Rifampicin and Clofazimine as multidrug therapy for the treatment of Mycibacterium leprae infections. It is also second-line treatment for prevention against Pneumocystis pneumonia caused by Pneumocystis jirovecii in HIV patients in whom CD4 counts are below 200/mm3.
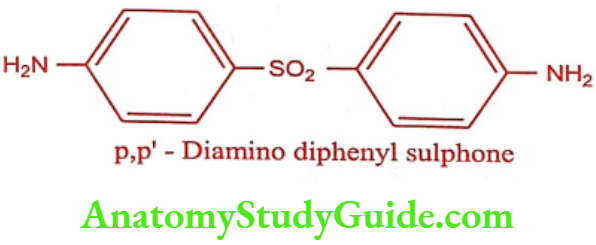
Synthesis
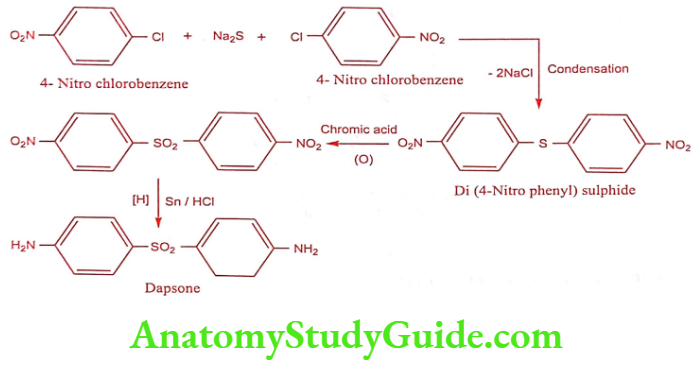
ADR: Anemia and peripheral neuropathy.
Dose: 100mg daily.
Use: It is used in the treatment of leprosy and nocardiosis.
Sulphonamide for Urinary Tract Infection
Sulphacetamide
Sulphadiazine
Trimethoprim (Proloprim, Monotrim)
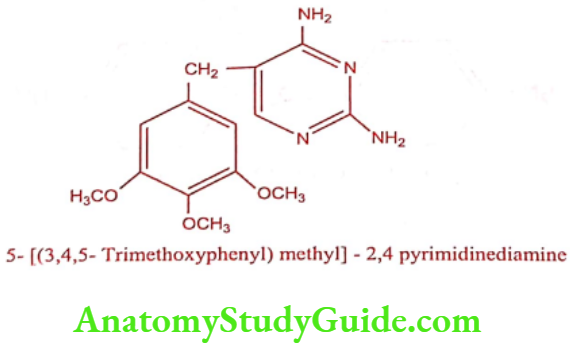
Synthesis
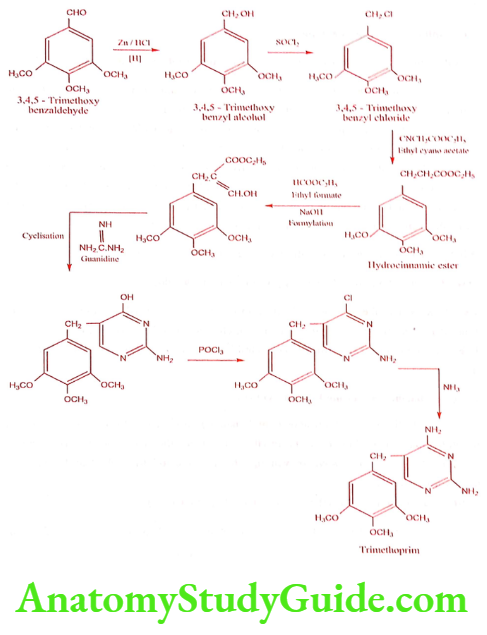
ADR: Agranulocytosis, hemolytic and megaloblastic anemia, thrombocytopenia and hypersensitivity reaction.
Dose: 200mg twice a day.
Use: It is used widely in the treatment of urinary tract, respiratory tract infection, and typhoid.
Sulphonamide Combinations
Sulphamethoxazole with Trimethoprim (Co-trimoxazole, Septran, Bactrim)
The synergistic effect is achieved by the combination of Trimethoprim and Sulphamethoxazole is widely used in antibacterial therapy. Bacteriostatic activity is obtained by two prominent steps in bacterial enzymatic pathway involved in folate synthesis.
Sulphamethoxazole inhibits utilization of PABA in the formation of dihydrofolate, while Trimethoprim is a selective inhibitor of the enzyme that catalyses the conversion of dihydrofolate to tetrahydrofolate. Sulphamethoxazole was selected from the systemic Sulphonamide on the basis that it has similar pharmacokinetic features to that of Trimethoprim. It is co-administered with Trimethoprim in a fixed dose ratio of 5:1.
ADR: Renal failure, nausea, vomiting, diarrhea, anorexia and skin rashes.
Dose: 960mg bid, up to 2.88gm daily given in two divided doses in severe cases.
Use: It is used in the treatment of infection of urinary, intestinal and lower respiratory tract. It is also effective in acute otitis media, chronic bacterial prostates, meningococcal infection, gonorrhea, nocardiosis and antibiotics resistant Salmonella and Shigella infections.
Sulphadiazine with Trimethoprim (Aubril)
Sulphadiazine is a short acting sulphonamide derivative with bacteriostatic action through competitive inhibition of bacterial synthesis of folic acid. Trimethoprim inhibits. enzymes folic acid pathway, preventing the reaction of the dihydrofolic acid to tetrahydrofolate.
ADR: Nausea, vomiting, diarrhea, anorexia and hypersensitivity.
Dose: Two tablets (410mg Sulphadiazine and 90mg Trimethoprim) as a single dose for every 24 hrs.
Use: It is used to treat urinary tract infection including those infections that affect the bladder like cystitis and polynephritis.
Sulphamoxole with Trimethoprim (Cortina, Supristol)
Supristol is a broad spectrum antibacterial agent, effective against Gram positive. and Gram negative organisms. Its action is based on the sequential blockade of two enzymes which act within the bacterial metabolic pathway of the biosynthesis of Folinic acid.
ADR: Nausea, vomiting, anorexia, diarrhea and hallucination.
Dose: Initially 2 tablets (400mg Sulphamoxole and 60mg Trimethoprim) followed by 1 tablet bid.
Use: It is used in systemic treatment of infections.
Sulphadoxine with Pyrimethamine (Malocide, Laridox)
Sulphadoxine is used in combination with Pyrimethamine. The Sulphadoxine interferes with the parasite ability to synthesis folic acid and Pyrimethamine inhibits the reduction of folic acid to its active tetrahydrofolate coenzyme form. The synergistic antifolate combination of Sulphadoxine a long acting sulphonamide with Pyrimethamine is used to treat malaria. The combination is considered as schizonticidal.
ADR: Urticaria, serum sickness, photosensitization and arthralgia.
Dose: 2 to 3 tablets (1500mg Sulphadoxine and 75mg Pyrimethamine) as a single dose.
Use: Sulphadoxine has antibacterial activity. In combination with Trimethoprim it is used as antibacterial agent with Pyrimethamine and Mefloquine it is used as antimalarial.
Leave a Reply