Discoloration Of Teeth Causes Treatment And Management Notes
Teeth Introduction
Teeth are polychromatic so color varies among the gingival, incisal, and cervical areas according to the thickness, reflections of different colors, translucency, the thickness of enamel and color of dentin.
Table of Contents
The thickness of enamel is greater at the occlusal/incisal third of the tooth and thinner at the cervical third. That is why teeth are more darker on cervical one third than at the middle or incisal one-third.
Read And Learn More: Endodontics Notes
Normal color of primary teeth is bluish-white, whereas color of permanent teeth is grayish yellow, grayish white, or yellowish white.
With age, the color of teeth changes to more yellow or grayish-yellow due to an increase in dentin thickness and a decrease in enamel thickness.
Classification Of Discoloration
Color of the teeth is inflenced by a combination of their intrinsic color and presence of any extrinsic stains on the tooth surface.
Intrinsic tooth color is associated with light scattering and adsorption properties of enamel and dentine, where dentine plays a major role in determining the overall shade.
Extrinsic stains form due to smoking, dietary intake of tannin-rich foods, use of some cationic agents like chlorhexidine, or metal salts like tin and iron.
So, discoloration of teeth can be classified as:
- Intrinsic discoloration
- Extrinsic discoloration
- Combination of both
Intrinsic Stains
Preeruptive Causes
These are incorporated into the deeper layers of enamel and dentin during odontogenesis and alter the development and appearance of the enamel and dentin.
Alkaptonuria
Dark brown pigmentation of primary teeth is commonly seen in alkaptonuria.
It is an autosomal recessive disorder resulting into the complete oxidation of tyrosine and phenylalanine causing increased level of homogentisic acid.
Etiology
Hematological Disorders
- Erythroblastosis fetalis: It is a blood disorder of neonates due to Rh incompatibility. In this, stain does not involve teeth or portions of teeth developing after cessation of hemolysis shortly after birth. Stain is usually green, brown, or bluish in color.
- Congenital porphyria: It is an inborn error of porphyrin metabolism, characterized by the overproduction of uroporphyrin. Deciduous and permanent teeth may show a red or brownish discoloration. Under ultraviolet light, teeth show red fluorescence.
- Sickle cell anemia: It is inherited blood dyscrasia characterized by increased hemolysis of red blood cells. In sickle cell anemia infrequently the stains of the teeth are similar to those of erythroblastosis fetalis, but the discoloration is more severe, involves both dentitions, and does not resolve with time.
Diseases of Enamel and Dentin.
- Amelogenesis imperfecta (AI): It comprises a group of conditions that demonstrate developmental alteration in the structure of the enamel in the absence of systemic disorders. AI has been classified mainly into hypoplastic, hypocalcemia, and hypomaturation type.
- Fluorosis: In fluorosis, staining is due to excessive fluoride uptake during the development of enamel. Excess fluoride induces a metabolic change in ameloblast and the resultant enamel has a defective matrix and an irregular, hypomineralized structure.
Fluorosis staining manifests as:
- Gray or white opaque areas on teeth
- Yellow to brown discoloration on a smooth enamel surface
- Moderate and severe changes show pitting and brownish discoloration of the surface
- Severely corroded appearance with dark brown discoloration and loss of most of the enamel
- Enamel hypoplasia and hypo calcification due to other causes:
- Vitamin D deficiency results in characteristic white patch hypoplasia in teeth
- Vitamin C deficiency together with vitamin A deficiency during formative periods of dentition results in a pitting-type appearance of teeth.
- Childhood illnesses during odontogenesis, such as exanthematous fevers, malnutrition, and metabolic disorder also affect teeth.
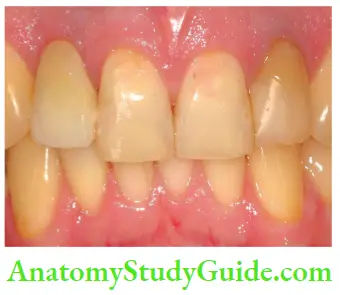
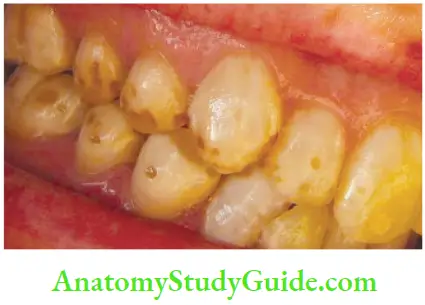
Dentinogenesis imperfect (DI):
- It is an autosomal dominant development disturbance of the dentin which occurs along or in conjunction with AI.
- The color of teeth in DI varies from gray to brownish violet to yellowish brown with a characteristic usual translucent or opalescent hue.
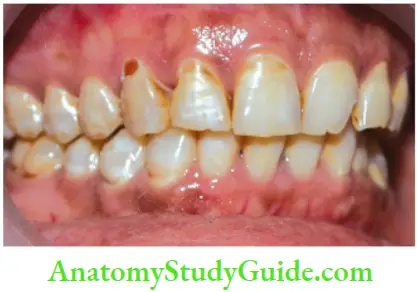
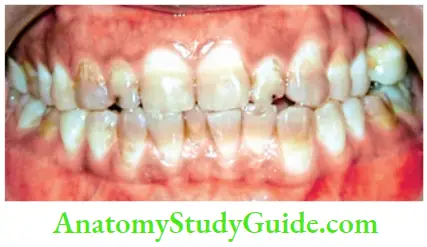
Tetracycline and minocycline:
- Unsightly discoloration of both dentitions result from excessive intake of tetracycline and minocycline during the development of teeth.
- Chelation of tetracycline molecule with calcium in hydroxyapatite crystals forms tetracycline orthophosphate which is responsible for discolored teeth.
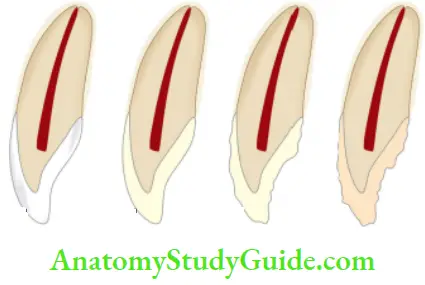
Classification of tetracycline staining according to developmental stage, banding, and color (Jordan and Boksman, 1984):
- First degree (mild)—yellow to gray, uniformly spread through the tooth. No banding
- Second degree (moderate)—yellow-brown to dark gray, slight banding, if present
- Third degree (severe staining)—blue-gray or black and is accompanied by significant banding across the tooth
- Fourth degree—stains that are so dark that bleaching is ineffective, totally
The severity of pigmentation with tetracycline depends on three factors:
- Time and duration of administrations
- Type of tetracycline administered
- Dosage
Posteruptive Causes
- Pulpal changes: Pulp necrosis usually results from bacterial, mechanical, or chemical irritation to a pulp. In this, disintegration products enter dentinal tubules and cause discoloration.
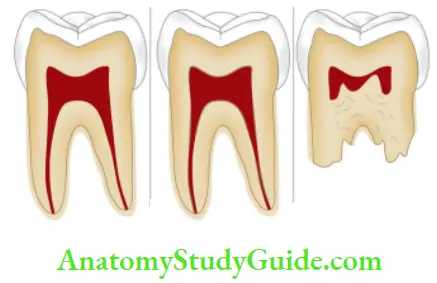
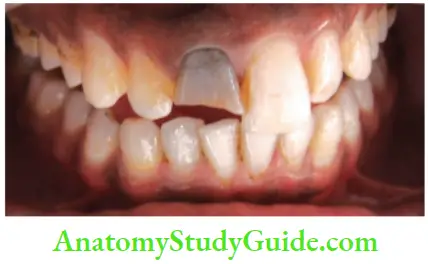
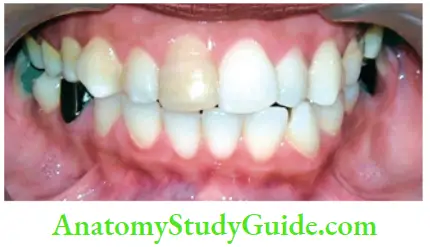
- Trauma: Accidental injury to the tooth can cause pulpal and enamel degenerative changes that may alter the color of teeth. Pulpal hemorrhage leads to grayish discoloration and nonvital appearance. Injury causes hemorrhage which results in lysis of RBCs and liberation of iron sulfide which enters dentinal tubules and discolors the surrounding tooth.
- Dentin hyper calcification: Dentin hyper calcification results when there are excessive irregular elements in the pulp chamber and canal walls. It causes a decrease in translucency and yellowish or yellow-brown discoloration of the teeth.
- Dental caries: In general, teeth present a discolored appearance around areas of bacterial stagnation and leaking restorations.
- Restorative materials and dental procedures: Discoloration can also result from the use of endodontic sealers and restorative materials
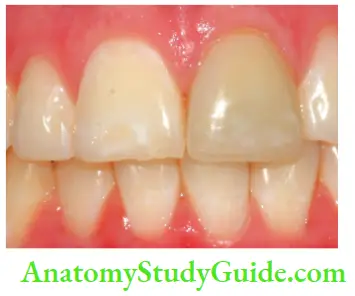
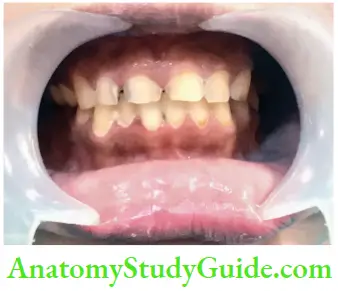
- Aging: Color changes in teeth with age result from surface and subsurface changes. Age-related discoloration is because of
- Enamel changes: Both thinning and texture changes occur in the enamel
- Dentin deposition: Secondary and tertiary dentin deposits and pulp stones cause changes in the color of teeth
- Functional and parafunctional changes: Tooth wear may give a darker appearance to the teeth because of loss of tooth surface and exposure of dentin which is yellower and is susceptible to color changes by absorption of oral fluids and deposition of reparative dentin.
Extrinsic Stains
Classification of Extrinsic Stains (Nathoo in 1997)
- N1 type dental stain (direct dental stain): Here colored materials bind to the tooth surface to cause discoloration. Tooth has same color, as that of chromogen
- N2 type dental stain (direct dental stain): Here chromogen changes color after binding to the tooth
- N3 type dental stain (indirect dental stain): In this type, prechromogen (colorless) binds to the tooth and undergoes a chemical reaction to cause a stain
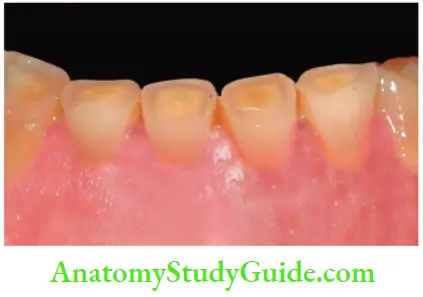
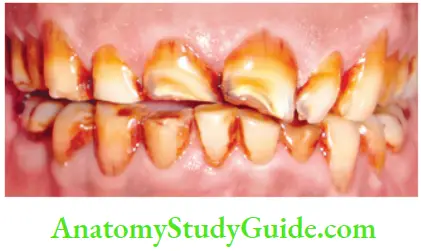
Daily Acquired Stains
- Plaque: Pellicle and plaque on tooth surface gives rise to yellowish appearance of teeth
- Food and beverages: Tea, coffe, red wine, curry, and colas if taken in excess cause discoloration
- Tobacco use: It results in brown to black appearance of teeth
- Poor oral hygiene manifests as:
- Green stain
- Brown stain
- Orange stain
- Swimmer’s calculus: It is yellow to dark brown stain present on facial and lingual surfaces of anterior teeth. It occurs due to prolonged exposure to pool water
- Gingival hemorrhage
Chemicals
- Chlorhexidine stain: The stains produced by use of chlorhexidine are yellowish-brown to brownish in nature
- Metallic stains: Thse are caused by metals and metallic salts introduced into the oral cavity in metal-containing dust inhaled by industry workers or through orally administered drugs
Stains caused by different metals
- Copper dust—green stain
- Iron dust—brown stain
- Mercury—greenish black stain
- Nickel—green stain
- Silver—black stain
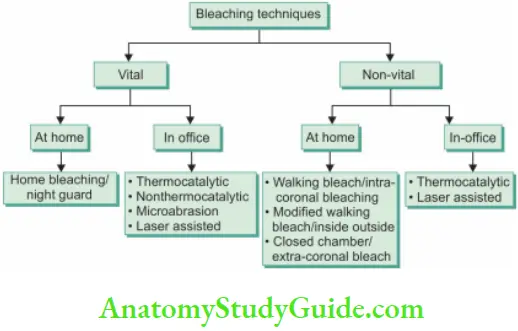
Bleaching
Bleaching is a procedure that involves lightening of the color of a tooth through the application of a chemical agent to oxidize the organic pigmentation in the tooth.
The goal of bleaching is to restore the normal color of a tooth by lightening the stain with a powerful oxidizing agent, also known as a bleaching agent.
Bleaching Mechanism
The mechanism of bleaching is mainly linked to the degradation of high molecular weight complex organic molecules that reflect a specific wavelength of light, which is responsible for the color of the stain.
Resulting degradation products are of lower molecular weight and composed of less complex molecules that reflect less light, resulting in a reduction or elimination of discoloration.
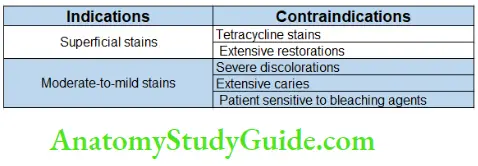
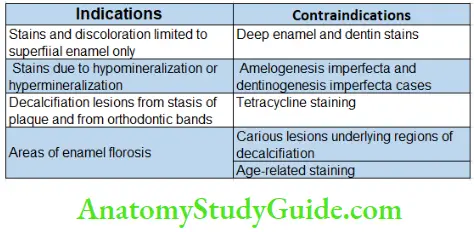
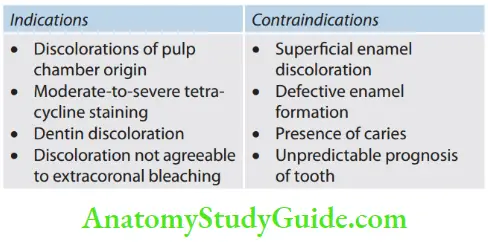
Bleaching Indications
- Generalized staining
- Age-related discolorations
- White spots
- Mild tetracycline staining
- Mild fluorosis without pitting
- Acquired superficial staining; dietary staining (tea/coffee)
- Stains from smoking tobacco
- Color changes related to pulpal trauma or necrosis.
Contraindications
Poor Case Selection
Patient having emotional or psychological problems is not the right choice for bleaching.
Dentin Hypersensitivity
Hypersensitive teeth need to provide extra protection before going for bleaching.
Extensively Restored Teeth
These teeth are not good candidates for bleaching because of insufficient enamel to respond properly to bleaching.
In teeth with large composite restorations, the restoration becomes more evident after bleaching.
Teeth with Hypoplastic Marks and Cracks
The application of bleaching agents increases the contrast between white opaque spots and normal tooth structure.
In these cases, bleaching can be done in conjunction with microabrasion, annuloplasty, and composite resin bonding.
Defective and Leaky Restorations
Defective and leaky restorations are not good candidates for bleaching. If discoloration is from metallic salts, particularly silver amalgam, dentinal tubules of the tooth become saturated with alloys and no amount of bleaching will significantly improve the shade.
Defective Obturation
If a root canal is not well-obturated, then refiling must be done before attempting bleaching.
Bleaching Agents
Different types of bleaching agents are available commercially. These bleaching agents may contain the following components.
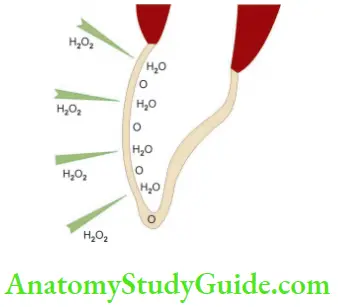
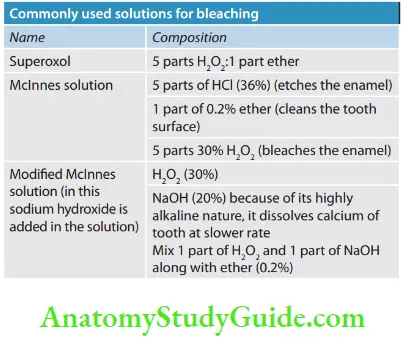
Hydrogen Peroxide
- Used in concentrations ranging from 5% to 35%
- H2O2 has low molecular weight so can penetrate dentin and release oxygen
- It is clear, colorless, odorless liquid stored in lightproof bottles
- If stored properly, its shelf life is 3–4 months but decomposes rapidly in the presence of organic debris and an open-air
- Should be handled carefully to prevent direct contact with mucous membrane
- Can be used alone or in combination with sodium perborate
Sodium Perborate
- Available as a white powder in granular form
- Mainly three types: sodium perborate monohydrate, trihydrate, and tetrahydrate, and these three types vary in oxygen content.
- When mixed with supercool, it decomposes into sodium metaborate, water, and oxygen
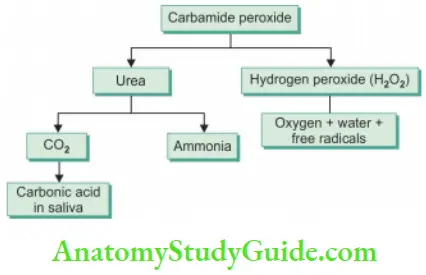
Carbamide Peroxide
- Also known as urea hydrogen peroxide
- Used in concentrations ranging from 3% to 45%
- It decomposes into urea, ammonia, carbon dioxide, and hydrogen peroxide
- Carbopol (polyacrylic acid polymer) is used as a thickening agent. It prolongs the release of active peroxide
- For gel preparations, glycerine, propylene glycol, sodium stannate, citric acid, and flavoring agents are added
Bleaching Techniques(Flow Chart)
Home Bleaching Technique
The home bleaching technique is also called night guard bleaching.
Commonly Used Solution for Night Guard Bleaching
-
- 10% carbamide peroxide with or without carpool
- 15% carbamide peroxide
- Hydrogen peroxide (1–10%)
Factors Affecting Prognosis
- History or presence of sensitive teeth
- An extremely dark gingival third of the tooth is visible during smiling
- Extensive white spots
- Translucent teeth
- Excessive gingival recession and exposed root surfaces
Steps Of Tray Fabrication
- Take the impression and make a stone model
- Trim the model
- Place the stock out of resin and cure it
- Apply separating media
- Choose the tray sheet material
- Nature of material used for the fabrication of the bleaching tray is flexible plastic. The most common tray material used is ethyl vinyl acetate
- Cast the plastic in vacuum tray forming machines
- Trim and polish the tray
- Checking the tray for correct fit, retention, and overextension
- Demonstrate the amount of bleaching material to be placed
Thickness of Tray
- Standard thickness of the tray is 0.035
- Thicker tray, that is, 0.05 in. is indicated in patients with breaking habit
- A thinner tray, that is, 0.02 in. thick is indicated in patients who gag
Treatment Regimen
- Patient is instructed to brush the teeth before tray application
- The patient is instructed to place enough bleaching material into the tray to cover the facial surfaces of the teeth.
- After seating the tray in the mouth, the extra material is carefully wiped away
- Wearing the tray during day time allows replenishment of the gel after 1–2 h for maximum concentration. Overnight use causes a decrease in loss of material due to decreased salivary flow at night
- While removing the tray, the patient is asked to remove the tray from the second molar region in a peeling action. This is done to avoid injury to soft tissues
- The patient is instructed to rinse of the bleaching agent and clean the tray
- Duration of treatment depends upon original discoloration, duration of bleaching, patient compliance, and time of bleaching
- Patient is recalled for periodic checkups for assessing the bleaching process.
Regimen Maintenance
Additional rebleaching can be done every 3–4 years if necessary with a duration of 1 week.
Regimen Side Effects
- Gingival irritation—painful gums after a few days of wearing trays
- Soft tissue irritation—from excessive wearing of the trays or applying too much bleach to the trays
- Altered taste sensation—metallic taste immediately after removing trays
- Tooth sensitivity—most common side effect
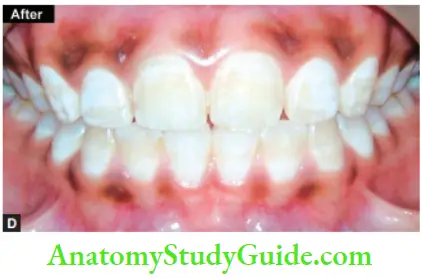
In-Office Bleaching
Thermocatalytic Vital Tooth Bleaching
Equipment Needed
- Power bleach material
- Tissue protector
- Energizing/activating source
- Protective clothing and eyewear
- Mechanical timer
Light Sources
- Conventional bleaching light
- Uses heat and light to activate bleaching material
- More heat is generated during bleaching
- Causes tooth dehydration
- Uncomfortable for patient
- Slower in action
- Tungsten halogen curing light
- Uses light and heat to activate the bleaching solution
- Application of light 40–60 s per application per tooth
- Time-consuming
Xenon plasma arc light
- High-intensity light, so more heat is liberated during bleaching
- The application requires 3 s per tooth
- Faster bleaching
- Action is thermal and stimulates the catalyst in chemicals
- Greater potential for thermal trauma to pulp and surrounding soft tissues
Argon and CO2 laser
- True laser light stimulates the catalyst in chemicals so there is no thermal effect
- Requires 10 s per application per tooth
Diode laser light
- True laser light produced from a solid state source
- Ultrafast
- Requires 3–5 s to activate bleaching agent
- No heat is generated during bleaching
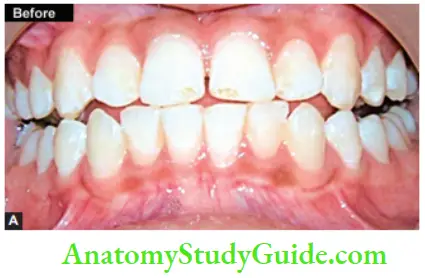
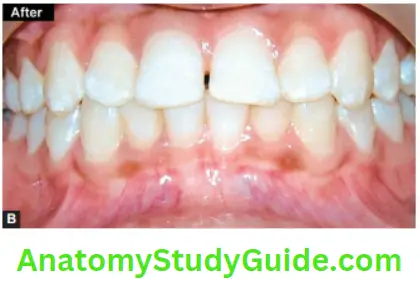
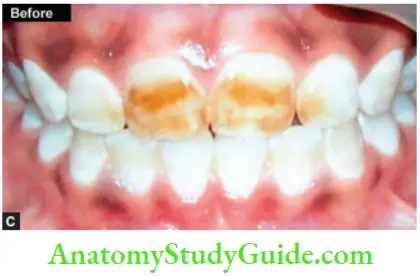
Procedure
- Pumice the teeth to clean of any debris present on the tooth surface
- Isolate the teeth with a rubber dam and protect the gingival tissues with orabase or vaseline. Protect the patient’s eyes with sunglasses
- Saturate the cotton or gauze piece with bleaching solution (30–35% H2O2) and place it on the teeth
- Depending upon light, expose the teeth. The temperature of the device should be maintained between 52 and 60°C (125–140°F)
- Change solution in between after every 4–5 min. The treatment time should not exceed 30 min
- Remove solution with the help of a wet gauge
- Remove the solution and irrigate teeth thoroughly with warm water
- Polish teeth and apply a neutral sodium fluoride gel
- Instruct the patient to use fluoride rinse on a daily basis
- Second and third appointment is given after 3–6 weeks. This will allow pulp to settle
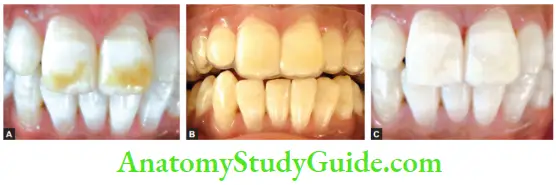
Image show bleaching of teeth by thermocatalytic vital bleaching technique.
Nonthermocatalytic Bleaching In this technique, the heat source is not used.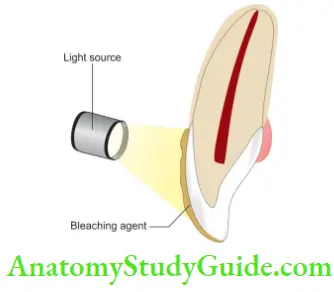
Steps
- Isolate the teeth using rubber dam
- Apply bleaching agent on the teeth for 5 min Thermocatalytic technique of bleaching for vital teeth.
- Wash the teeth with warm water and reapply the bleaching agent until the desired color is achieved
- Wash the teeth and polish them
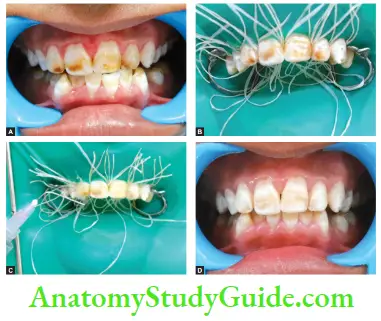
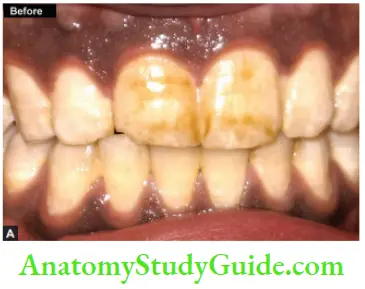
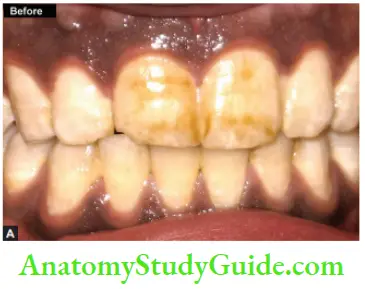
Microabrasion
It is a procedure in which a microscopic layer of enamel is simultaneously eroded and abraded with a special compound (usually contains 18% of hydrochloric acid) leaving a perfectly intact enamel surface behind.
Protocol
- Clinically evaluate the teeth
- Clean teeth with rubber cup and prophylaxis paste
- Apply petroleum jelly to the tissues and isolate the area with rubber dam
- Apply microabrasion compound to areas in 60-s intervals with appropriate rinsing
- Repeat the procedure if necessary. Check the teeth when wet
- Rinse teeth for 30 s and dry
- Apply topical fluoride to the teeth for 4 min
- Re-evaluate the color of the teeth. More than one visit may be necessary sometimes

Bleaching of Nonvital Teeth
- Thermocatalytic Technique of Bleaching for Nonvital Teeth
- Isolate the tooth to be bleached using rubber dam
- Place the bleaching agent (superoxide and sodium perborate separately or in combination) in the tooth chamber
- Heat the bleaching solution using a bleaching stick/light curing unit
- Repeat the procedure till the desired tooth color is achieved
- Wash the tooth with water and seal the chamber using dry cotton and temporary restorations
- Recall the patient after 1–3 weeks
- Do the permanent restoration of tooth using suitable composite resins afterward
- Intracoronary Bleaching/Walking Bleach Of Nonvital Teeth
It involves use of chemical agents within the coronal portion of an endodontically treated tooth to remove tooth discoloration.
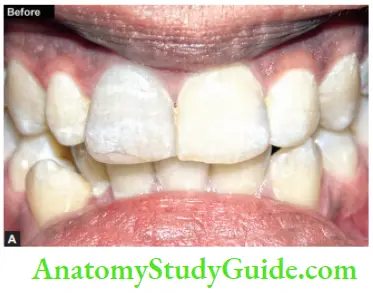
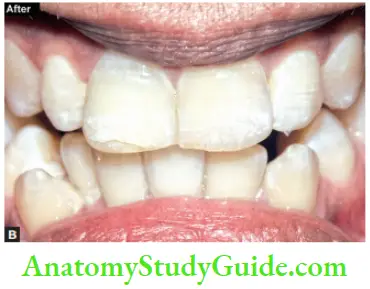
Steps
- Take radiographs to assess the quality of obturation. If found unsatisfactory, retreatment should be done
- Evaluate the quality and shade of restoration, if present. If restoration is defective, replace it
- Evaluate tooth color with shade guide
- Isolate the tooth with a rubber dam
- Prepare the access cavity, remove the coronal gutta-percha, expose the dentin, and refine the cavity
- Place mechanical barriers of 2 mm thick, preferably of glass ionomer cement, zinc phosphate, IRM polycarboxylate cement, or MTA on root canal filing material. The coronal height of the barrier should protect the dentinal tubules and conform to the external epithelial attachment
- Now mix sodium perborate with an inert liquid (local anesthetic, saline, or water) and place this paste into a pulp chamber. In case of severe stains add 3% hydrogen peroxide to make a paste
- After removing the excess bleaching paste, place a temporary restoration over it. Apply pressure with the gloved figer against the tooth until the filling has set because the filing may get displaced due to the release of oxygen
- Recall the patient after 1–2 weeks, and repeat the treatment until desired shade is achieved
Restore access cavity with composite after 2 weeks
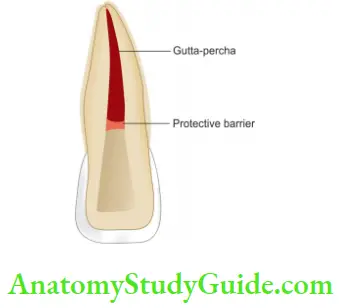
Complications of Intracoronal Bleaching
- External root resorption
- Chemical burns if using 30–35% H2O2 so gingival should be protected using petroleum jelly or cocoa butter.
- Decrease bond strength of composite because of the presence of residual oxygen following the bleaching procedure
Sodium ascorbate is a buffered form of vitamin C which consists of 90% ascorbic acid bound to 10% sodium. It is a powerful antioxidant used for the removal of residual oxygen after bleaching.
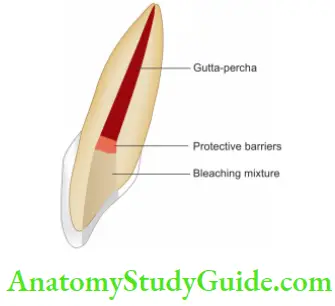
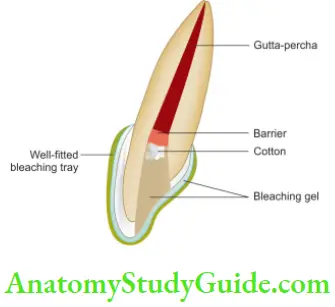
- Inside/Outside Bleaching Technique
Bleaching Technique Synonyms
Internal/external bleaching, modified walking bleach technique.
This technique involves intracoronal bleaching technique along with the home bleaching technique.
This is done to make the bleaching program more effective. This combination of bleaching treatments is helpful in treating difficult stains, for specific problems like single dark vital or nonvital tooth and to treat stains of different origins present on the same tooth.
Bleaching Technique Procedure
- Assess the obturation by taking radiographs
- Isolate the tooth and prepare the access cavity by removing gutta-percha 2–3 mm below the cementoenamel junction
- Place the mechanical barrier, clean the access cavity and place a cotton pellet in the chamber to avoid food packing into it
- Evaluate the shade of the tooth
- Check the fitting of the bleaching tray and advise the patient to remove the cotton pellet before bleaching
- Instructions for home bleaching. A bleaching syringe can be directly placed into the chamber before seating the tray or extra bleaching material can be placed into the tray space corresponding to the tooth with an open chamber
- After bleaching, tooth is irrigated with water, cleaned, and again a cotton pellet is placed in the empty space
- Reassessment of shade is done after 4–7 days
- When the desired shade is achieved, seal the access cavity initially with temporary restoration and finally with composite restoration after ≥2 weeks
Bleaching Technique Advantages
- More surface area for bleach to penetrate
- Treatment time in days rather than weeks
- Decreases the incidence of cervical resorption
- Uses a lower concentration of carbamide peroxide
Bleaching Technique Disadvantages
- Noncompliant patients
- Overbleaching by overzealous application
- Chances for cervical resorption is reduced but still exist
- Closed Chamber Bleaching/Extracoronal Bleaching
In this technique, instead of removing the existing restoration, the bleaching paste is applied to the tooth via a bleaching tray.
- Indications of Closed Chamber Technique
- In case of totally calcified canals in a traumatized tooth
- As a maintenance bleaching treatment several years after initial intracoronal bleaching
- Treatment for adolescents with incomplete gingival maturation
- A single dark nonvital tooth where the surrounding teeth are sufficiently light or where other vital teeth are also to be bleached
Laser-Assisted Bleaching Technique
Ths technique achieves a power bleaching process with the help of effient energy source with minimum side effects.
The laser whitening gel contains thermally absorbed crystals, fumed silica, and 35% H2O2.
In this, the gel is applied and is activated by a light source which in further activates the crystals present in gel, allowing the dissociation of oxygen and therefore better penetration into enamel matrix.
Following lasers have been approved by FDA for tooth bleaching:
- Argon laser
- CO2 laser
- GaAlAs diode laser.
Argon Laser
- It Emits a wavelength of 480 nm in the visible part of spectrum
- Activates the bleaching gel and makes the darker tooth surface lighter
- Less thermal effcts on pulp as compared to other heat lamps.
CO2 Laser
- Used to enhance the effect of whitening produced by argon laser
- Deeper penetration than argon laser thus more efficient tooth whitening
- More deleterious effects on pulp than argon laser
GaAlAs Diode Laser (Gallium-Aluminum-Arsenic)
Emits a wavelength of 980 nm.
Effects of Bleaching Agents on Tooth and its Supporting Structures
Tooth Hypersensitivity
Tooth sensitivity is a common side effect of external tooth bleaching. Higher incidences of tooth sensitivity (67–78%) are seen after in-office bleaching with hydrogen peroxide in combination with heat.
The th mechanism responsible for external tooth bleaching though is not fully established, but it has been shown that peroxide penetrated enamel, dentin, and pulp.
This penetration was more in restored teeth than that of intact teeth.
Effects on Enamel
Studies have shown that 10% carbamide peroxide significantly decreased enamel hardness. But the application of fluoride showed improved remineralization after bleaching.
Effects on Dentin
Bleaching has been shown to cause uniform changes in color through dentin.
Effects on Pulp
Penetration of bleaching agent into pulp through enamel and dentin occurs resulting in tooth sensitivity.
Studies have shown that 3% solution of H2O2 can cause transient reduction in pulpal blood flow and occlusion of pulpal blood vessels.
Effects on Cementum
Recent studies have shown that cementum is not affected by materials used for home bleaching. But cervical resorption and external root resorption in teeth has been seen in teeth treated by intracoronal bleaching using 30–35% H2O2.
Cervical Resorption
More serious side effects such as external root resorption may occur when a higher than 30% concentration of hydrogen peroxide is used in combination with heat.
Hydroxyl groups may be generated during thermocatalytic bleaching, especially where ethylenediamine tetraacetic acid has been used previously to clean the tooth.
Hydroxyl ions may stimulate cells in the cervical periodontal ligament to differentiate into odontoclasts, which begin root resorption in the area of the tooth below the epithelial attachment.
Mucosal Irritation
A high concentration of hydrogen peroxide (30–35%) is caustic to mucous membranes and may cause burns and bleaching of the gingiva.
Genotoxicity and Carcinogenicity
Hydrogen peroxide shows a genotoxic effect as free radicals released from hydrogen peroxide (hydroxyl radicals, per hydroxyl ions and superoxide anions) are capable of attacking DNA.
Toxicity
The acute effects of hydrogen peroxide ingestion are dependent on the amount and concentration of hydrogen peroxide solution ingested.
Signs and symptoms usually seen are ulceration of the buccal mucosa, esophagus, and stomach, nausea, vomiting, abdominal distention, and sore throat.
Bleaching is safe, economical, conservative, and effective method of decoloring stained teeth due to various reasons.
It should always be given thought before going for more invasive procedures like veneering or full ceramic coverage, depending upon specific case.
Effects on Restorative Materials
Application of bleaching on composites has shown the following changes:
- Increased surface hardness
- Surface roughening and etching
- Decrease in tensile strength
- Increased microleakage
- No signifiant color change of composite material itself other than the removal of extrinsic stains around existing restoration
Bleaching Conclusion
Bleaching is a safe, economical, conservative, and effective method of decoloring stained teeth due to various reasons.
It should always be given thought before going for more invasive procedures like veneering or full ceramic coverage, depending upon a specific case.
It can be performed in the office or at home as per the patient’s requirements. However, as with any dental procedure, bleaching involves risks.
Clinicians should inform their patients about the possible changes that may occur on their dental tissues and restorations after the bleaching procedure so as to compare risks versus benefit of the procedure.
Leave a Reply